Hashimoto Encephalopathy: Encephalitis versus Vasculopathy and the Impact of Concussion
David S Younger
DOI10.21767/2471-8513.100054
David S Younger*
Department of Neurology, New York University Langone Health, and the School of Medicine, New York University, New York, USA
- *Corresponding Author:
- David S Younger
Department of Neurology, New York University Langone Health
and the School of Medicine, New York University, New York, USA
E-mail: David.Younger@nyumc.org
Received date: February 05, 2018; Accepted date: February 19, 2018; Published date: February 24, 2018
Citation: Younger DS (2018) Hashimoto Encephalopathy: Encephalitis versus Vasculopathy and the Impact of Concussion. J Autoimmune Disord Vol 4:2. doi: 10.21767/2471-8153.100054
Copyright: © 2018 Younger DS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Patients with Hashimoto encephalitis may present with seizures, stroke-like episodes, transient focal and global neurological deficits, and a variety of neuropsychiatric disturbances. The encephalopathy evolves with elevated anti-thyroid peroxidase antibodies, independent of hormonal thyroid function. A vasculitic basis is considered in cases that show inflammatory vasculopathy in brain biopsy and postmortem tissues. A relation to autoimmune encephalitis is supported by etiologic localization of the disturbance to vulnerable areas of the mesial temporal lobe and the association of potentially pathogenic antibodies. Both mechanisms presume disruption of the blood-brain barrier. We report a patient with Hashimoto encephalopathy in whom repeated concussions antedated onset of the disorder with overlapping areas of altered brain metabolism and vascular perfusion in the hippocampus, deemed to be most vulnerable in autoimmune encephalopathy and concussive brain injury.
Keywords
Autoimmune; Encephalitis; Hashimoto; Encephalopathy; Vasculitis
Introduction
Hashimoto thyroiditis and encephalopathy is extensively reviewed by Younger [1]. Neurologists have been pursuing the associated but rare encephalopathy associated with Hashimoto thyroiditis for over fifty years. In 1966, the British neurologist, Lord Brain and colleagues [2] described Hashimoto encephalopathy in a 40-year-old man with ictal and stroke-like episodes of confusion and agitation one year after onset of treated hypothyroidism, eventually resolving with the likeliest explanation of an antibody-mediated autoimmune etiopathogenesis. Jellinek and Ball [3] extended the patient of Brain and colleagues [2], who later died at age 62, died 12 years after an unrelated cause. Autopsy showed no remaining thyroid tissue and atheromatous cerebrovascular changes with splenic atrophy. The authors [3] postulated an autoimmune disturbance as the cause of thyroiditis, encephalopathy, and splenic atrophy. In 2003, Rowland and colleagues [4] described the clinicopathologic findings of all literature cases of Hashimoto encephalopathy beginning with the patient described by Lord Brain and coworkers [2], and adding their own patient.
The diagnosis of Hashimoto encephalopathy rests upon the presence of thyroiditis, with measurably high titers of thyroid peroxidase (TPO) or thyroglobulin (Tg) antibodies, clinical encephalopathy (as evidenced by clouding of consciousness with reduced wakefulness, attention, or cognitive function), and absent evidence of cerebrospinal fluid (CSF) bacterial or viral infection [4]. Historically, as now, it is unknown whether anti-thyroid antibodies and thyroid dysfunction contribute to the pathogenesis of encephalopathy.
New York University Langone Health Case
The author found a recent case who met the criteria for Hashimoto encephalopathy as described by Rowland and colleagues [4], but with repeated concussive head injuries antedating Hashimoto encephalopathy.
For several years, a 19 year-old male with no previous history of psychiatric illness sustained several concussions associated with a sleigh ride, basketball, and hazing in boarding school. followed by cognitive and neurobehavioral impairments suggesting limbic encephalitis. Symptoms and signs of progressive apathy, anhedonia, lack of decision-making and goal-directed behavior, extreme fatigue, attention and memory impairment, irritability, severely disrupted sleep cycles, and increased eating with significant weight gain first brought him to medical attention. Psychiatric treatment modalities and behavioral modification were ineffective.
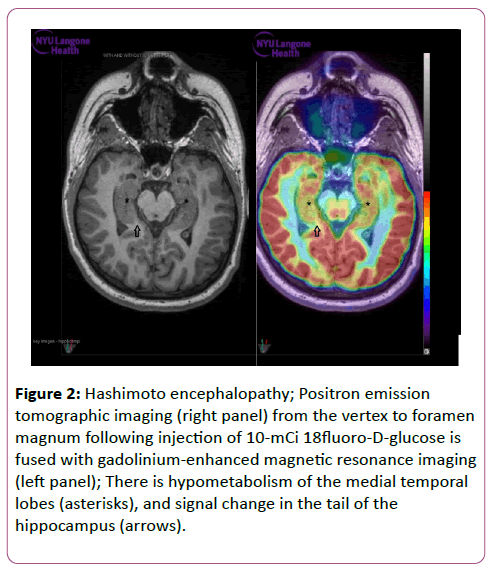
Examination in December 2016 showed a depressed mood with otherwise normal mental status. There was stocking sensory loss, mild distal weakness, hyporeflexia, and intact cranial nerves. Serum TPO and ATA antibodies were 35.4 IU/ml (reference value, <5 IU/ml) and 62.4 IU/ml (reference value, <5.5 IU/ml) respectively, with normal thyroid stimulating hormone and free T4 levels. Electrodiagnostic studies showed mild distal demyelinating and axonal changes. Epidermal nerve fiber (ENF) studies of the left calf and thigh showed significant distal small fiber neuropathy based on the pattern of reduced ENFs. Autonomic studies showed symptomatic orthostatic intolerance with a minimal systolic blood pressure of 90 millimeters (mm) of Hg (falling 20 mmHg from baseline), and a maximal heart rate of 115 beats per minute (bpm) (increasing 40 beats bpm from baseline) after 5 min of head-up tilting. Non-contrast magnetic resonance imaging (MRI) fused to 18fluorodeoxyglucose (FDG) positron emission tomography (PET) showed signal abnormality in the left hippocampus tail with hypometabolism in bilateral temporal and parietal lobes (Figure 1). Nuclear medicine cerebral perfusion single photon emission computed tomography (SPECT) showed hypoperfusion in bilateral temporal and right parietal lobes. Lumbar CSF analysis was normal without evidence of infection.
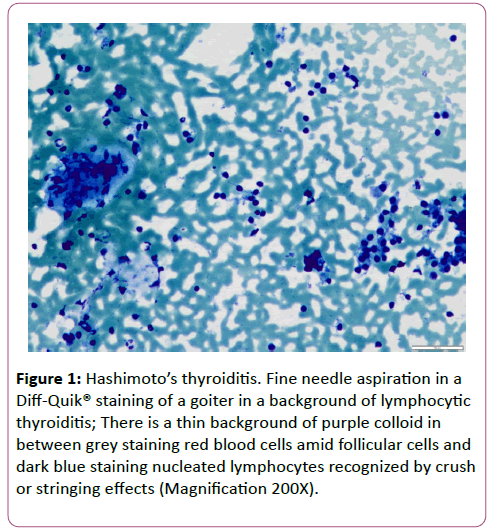
Figure 1: Hashimoto’s thyroiditis. Fine needle aspiration in a Diff-Quik® staining of a goiter in a background of lymphocytic thyroiditis; There is a thin background of purple colloid in between grey staining red blood cells amid follicular cells and dark blue staining nucleated lymphocytes recognized by crush or stringing effects (Magnification 200X).
Serum and CSF forwarded to Mayo Laboratories (Rochester, Minnesota) showed negative N-methyl-D-aspartate receptor (NMDA-R), voltage-gated potassium (VGKC)-complex, glutamic acid decarboxylase (GAD)-65, g-aminobutyric acid (GABA)-B-R, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)- R, anti-neuronal nuclear antibody (ANNA)-1, -2, -3; anti-glial neuronal antibody (AGNA)-1, Purkinje cell antibody (PCA)-1, -2, - Tr; amphiphysin, and collapsin response-mediator protein- (CRMP)-5-IgG antibodies. Similarly, serum Ma-1 and Ma-2 antibodies forwarded to Athena Diagnostics (Worcester,MA) was negative. Ultrasound of the thyroid showed a heterogeneous echotexture and micronodular pattern. Fine needle aspiration of the thyroid showed lymphocytic thyroiditis, Bethesda class II (Figure 2). Treatment with weekly intravenous immune globulin for nine months followed by a tapering three-month course of alternate day prednisone beginning at 60 mg alternate days led to significant improvement.
Figure 2: Hashimoto encephalopathy; Positron emission tomographic imaging (right panel) from the vertex to foramen magnum following injection of 10-mCi 18fluoro-D-glucose is fused with gadolinium-enhanced magnetic resonance imaging (left panel); There is hypometabolism of the medial temporal lobes (asterisks), and signal change in the tail of the hippocampus (arrows).
Discussion
The relation of Hashimoto encephalopathy to vasculitis was postulated in two cases studied histologically at autopsy showing lymphocytic infiltration of brainstem veins [5], while brain biopsy tissue of another patient showed lymphocytic infiltration of the walls of cerebral arterioles and veins [6]. Brain biopsy tissue in the patient described by Rowland and coworkers [4] showed perivascular cuffs of lymphocytic cells [4].
Ochi and colleagues [7] associated Hashimoto encephalopathy-related pathology and corticosteroid sensitivity with the novel antigen, -enolase, encoded on 1p36.23. Kishitani and coworkers [8] reported anti-NH2-terminal of -enolase antibodies in 24% of Hashimoto encephalopathy patients’ sera, and identified unilateral and bilateral mesial temporal lobe signal abnormalities on brain MRI that were associated with epileptic foci, suggesting a relation to limbic (autoimmune) encephalitis. Although antibodies to -enolase were not determined, signal abnormality in the tail of the hippocampus, and hypometabolism of the mesial temporal lobes in FDG PET-MRI in the present case supports a similar mechanism. Graus and colleagues [9] proposed categorization of such cases as autoimmune encephalitis after exclusion of other syndromes associated with well-defined autoantibodies. The relatively few biopsy and postmortem examinations available in patients with pathogenic serum antibodies show infiltrating inflammatory T-cells with cytotoxic granules in close apposition to hippocampal neurons, analogous to microscopic vasculitis [10].
The occurrence of repeated concussions in the present case is intriguing. The mechanisms underlying concussion, mild traumatic brain injury (mTBI) and chronic traumatic encephalopathy are closely associated with blood-brain barrier (BBB) disruption, metabolic and morphological brain alterations with activation of triggering receptor expressed on myeloid cells (TREM2), monocyte infiltration and phosphorylated axonal tauopathy [11]. Overlapping abnormalities on SPECT and PET imaging in the present case suggests BBB disruption that might have facilitated passage of pathogenic antibodies to vulnerable areas of the brain notably the mesial temporal lobe. Closedhead impact injury in experimental animals is associated with impaired axonal conduction in bilateral mesial hippocampal cells of the stratum alveus [11].
Treatment
The significance of corticosteroid sensitivity in Hashimoto encephalopathy is widely accepted as a criterion for the diagnosis. Patients with Hashimoto encephalopathy improve in association with, but not necessarily due to corticosteroid therapy. The present patient was treated with levothyroxine, corticosteroids, and immune modulatory therapy. However, it is not know to what degree, they affected coexisting inflammatory vasculopathy, autoimmune encephalopathy and the sequela of mTBI.
Conclusion
The terms Hashimoto thyroiditis and encephalopathy are deeply entrenched in the scientific literature. While the former is now well understood and a cornerstone for understanding autoimmune thyroid disease, the latter remains a challenge to clinicians, not because of its lethal nature, but because of the potential for unraveling an important feature of autoimmune encephalopathy, inflammatory vasculopathy, and mediating factors that lead to its expression. Patients who sustain concussive head injury and mTBI may be at greater risk for developing forms of autoimmune encephalopathy by allowing passage of circulatory cytokines and autoantibodies across a disrupted BBB.
References
- Younger DS (2017) Hashimoto thyroiditis and encephalopathy. World J Neurosci 7: 307-326.
- Brain L, Jellinek EH, Ball K (1996) Hashimoto’s disease and encephalopathy. Lancet 2: 512-514.
- Jellinek EH, Ball K (1976) Hashimoto’s disease, encephalopathy, and splenic atrophy. Lancet 1: 1248.
- Chong JY, Rowland LP, Utiger RD (2003) Hashimoto encephalopathy. Syndrome or myth? Arch Neurol 60: 164-171.
- Nolte KW, Unbehaun A, Sieker H, et al. (2000) Hashimoto encephalopathy: A brainstem encephalitis? Neurology 54: 769-770.
- Shibata N, Yamamoto Y, Sunami N, et al. (1992) Isolated angiitis of the CNS associated with Hashimoto's disease. Rinsho Shinkeigaku 32: 191-198.
- Ochi H, Horiuchi I, Araki N, et al. (2002) Proteomic analysis of human brain identifies -enolase as a novel autoantigen in Hashimoto’s encephalopathy. FEBS Lett 528: 197-202.
- Kishitani T, Matsunaga A, Masamichi I, et al. (2017) Limbic encephalitis associated with anti-NH2-terminal of ÃÆïÃâÃÂÃâáÃÆïÃâââ¬ÃâÃÂenolase antibodies. A clinical subtype of Hashimoto encephalopathy. Medicine 96: e6181.
- Graus F, Titulaer MJ, Balu R, et al. (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15: 391-404.
- Bien CG, Vincent A, Barnett MH, et al. (2012) Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135: 1622-1638.
- Tagge CA, Fisher AM, Minaeva OV, et al. (2018) Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141: 422-458.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences