Implications of Epigenetics in Myasthenia Gravis
Sujin Bao, Julie Rajotte-Caron, Danil Hammoudi and Jing Lin
Sujin Bao1*, Julie Rajotte-Caron1, Danil Hammoudi2 and Jing Lin3
1Saint James School of Medicine, Saint Vincent and the Grenadines
2Sinoe Medical Association, Baltimore, USA
3Icahn School of Medicine at Mount Sinai, USA
- *Corresponding Author:
- Sujin Bao
Department of Biochemistry
Saint James School of Medicine
Kingstown, Saint Vincent and the Grenadines
Tel: 7845267554
E-mail: baosujin@gmail.com
Received date: July 25, 2016; Accepted date: August 17, 2016; Published date: August 20, 2016
Citation: Bao S, Rajotte-Caron J, Hammoudi D, Lin J (2016) Implications of Epigenetics in Myasthenia Gravis. J Autoimmune Disord 2:3. doi: 10.21767/2471-8513.100022
Copyright: © 2016 Bao S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Myasthenia gravis is an autoimmune disease characterized by muscle weakness. The concordance rate of monozygotic twins in myasthenia gravis is about 30-40%, significantly higher than that in dizygotic twins, indicating genetic factors play a role in the pathogenesis of the autoimmune disorder. On the other hand, the concordance rate of the monozygotic twin is far below 100%, supporting the notion that environmental factors also play a role in the progression of the disorder. It is widely believed that environmental factors will influence the disease progression by altering gene expression. A long standing question is how the environment alters gene expression in humans. This review summarizes our current understanding of known mechanisms by which environmental factors such as medications, pollutants, food, sunlight, bacteria and viruses alter gene expression in some common autoimmune disorders. In this review, we also provide some literature evidence that supports epigenetics is a link between environment and abnormal gene expression in myasthenia gravis.
Keywords
Myasthenia gravis; Epigenetics; Genetics; Diagnosis; Treatment; Autoimmunity; Acetylcholine; Autoantibody
Introduction
Myasthenia gravis is an autoimmune disease. Patients with myasthenia gravis generally have weakness in skeletal and extraocular muscles with fatigue. The disorder is progressive and it often starts with a mild form (ocular form), in which weakness occurs only in specific muscles, most often, around the eye. The condition may become more severe with weakness seen in the extremities (generalized form). The name, myasthenia gravis, has both Greek and Latin roots. ‘Myasthenia’, derived from Greek, represents muscle weakness while ‘gravis’ in Latin stands for heavy or grievous [1]. The condition can occur in all races, both genders and at any age.
Molecular basis of myasthenia gravis
Among a vast majority of cases characterized to date, myasthenia gravis is caused by production of autoantibodies against own proteins at the postsynaptic neuromuscular junction. The major autoantibody found in myasthenia gravis recognizes acetylcholine receptor (AChR) [2]. The other antibodies involved in this condition may target muscle specific kinase (MuSK) or lipoprotein receptor related protein 4 (LRP4) [3].
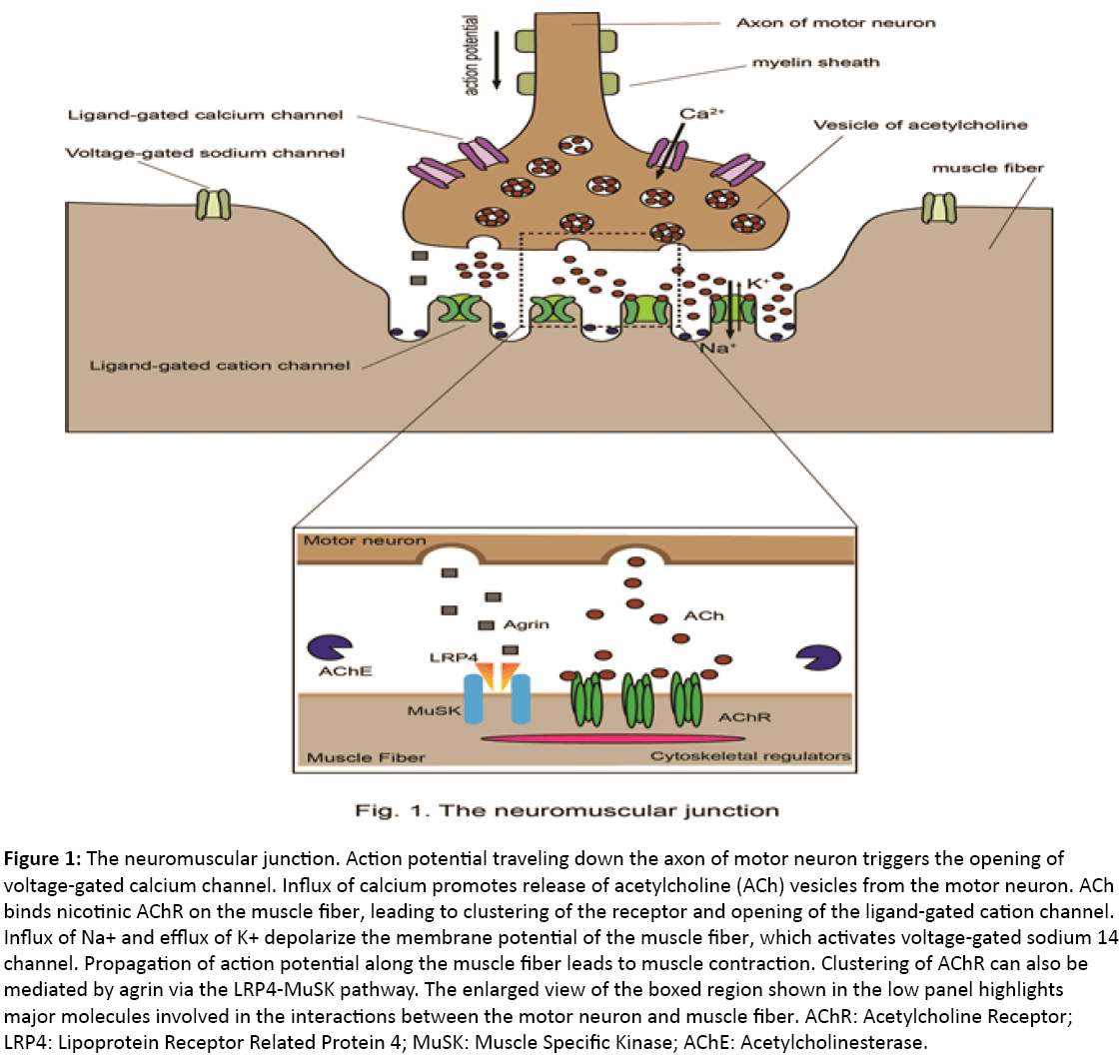
The neuromuscular junction is a chemical synapse that forms at the contact site between a motor neuron and a muscle fiber. Through the neuromuscular junction, motor neurons can transmit signals to the muscle. Often the direct outcome of the neural signal transmission is muscle contraction. Upon receiving an action potential, the presynaptic terminal of a motor neuron releases the neurotransmitter acetylcholine into the synaptic cleft (Figure 1). Acetylcholine diffuses and binds nicotinic acetylcholine receptor (AChR) on the postsynaptic membrane of the muscle cell. Nicotinic AChR is a ligand-gated ion channel that allows Na+ and K+ to pass through the membrane upon activation. The resulting depolarization opens a voltage-gated sodium channel that generates an action potential traveling along the muscle, leading to muscle contraction [4]. A key feature of the neuromuscular junction is that AChR form clusters. With AChR clusters, AChR is packed at a high density (about 10,000 molecules/μm2), which is important for rapid signal transmission between neurons and muscle fibers [5]. Musclespecific kinase (MuSK) is a master regulator of the AChR clustering and formation of the neuromuscular junction [6]. MuSK is a receptor tyrosine kinase and, not surprisingly, it interacts with a wealth of proteins.
Figure 1: The neuromuscular junction. Action potential traveling down the axon of motor neuron triggers the opening of voltage-gated calcium channel. Influx of calcium promotes release of acetylcholine (ACh) vesicles from the motor neuron. ACh binds nicotinic AChR on the muscle fiber, leading to clustering of the receptor and opening of the ligand-gated cation channel. Influx of Na+ and efflux of K+ depolarize the membrane potential of the muscle fiber, which activates voltage-gated sodium 14 channel. Propagation of action potential along the muscle fiber leads to muscle contraction. Clustering of AChR can also be mediated by agrin via the LRP4-MuSK pathway. The enlarged view of the boxed region shown in the low panel highlights major molecules involved in the interactions between the motor neuron and muscle fiber. AChR: Acetylcholine Receptor; LRP4: Lipoprotein Receptor Related Protein 4; MuSK: Muscle Specific Kinase; AChE: Acetylcholinesterase.
Among them, the secreted protein agrin is a ligand for the MuSK pathway and the membrane protein low-density lipoprotein receptor-related protein 4 (LRP4) is a co-receptor for agrin. LRP4 but not MuSK binds agrin directly. At the same time, LRP4 binds MuSK. In the cytoplasm, MuSK binds cytoskeletal regulators such as disheveled, Abl, Src homologous collagen D, Dok7 and Rapsyn (Figure 1). Through these cytoskeletal regulators, MuSK controls AChR clustering. So far, autoantibodies seen in myasthenia gravis interfere with signal transmission in the neuromuscular junction. Especially, Anti-MuSK and anti-LRP4 antibodies block AChR clustering while anti-AChR antibodies directly block the function of AChR in the muscle.
Cellular basis of myasthenia gravis
In a healthy individual, production of antibody is well controlled. How do patients develop autoantibodies against their own proteins?
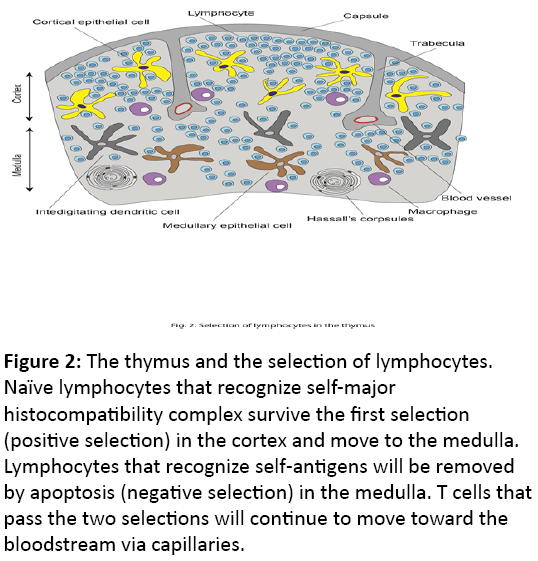
Antibodies are produced by plasma cells that are originally derived from B cells [7]. B cells that recognize and bind a specific foreign antigen will be activated and start to proliferate. Activation of most B cells requires the signals from helper T cells. Training and selection of helper T cells occur in the thymus in two steps. In the first step, naïve T cells that recognize self-major histocompatibility complex (self-MHC) proteins will survive in the thymic cortex (positive selection) and pass to the medulla. In the second step, developing T cells that recognize the self-antigen bound to self-MHC will be selected to die (negative selection). Only those T cells that recognize self-MHC but not self-antigens will survive and move on to blood circulation after passing capillaries (Figure 2).
Figure 2: The thymus and the selection of lymphocytes. Naïve lymphocytes that recognize self-major histocompatibility complex survive the first selection (positive selection) in the cortex and move to the medulla. Lymphocytes that recognize self-antigens will be removed by apoptosis (negative selection) in the medulla. T cells that pass the two selections will continue to move toward the bloodstream via capillaries.
So far, it is clear that myasthenia gravis is directly caused by defects in either B cells, T cells or both. Involvement of B cells in myasthenia gravis has been observed in thymic follicular hyperplasia. Normally germinal centers where most developing B cells reside during maturation are mainly found in peripheral lymphoid organs and only very few of them are found in the thymus. In thymic follicular hyperplasia, ectopic germinal centers are seen in the thymus. Patients with thymic follicular hyperplasia frequently develop anti-AChR antibodies [8]. Significantly, B cells from the thymus of myasthenia gravis patients are already activated and do not require other factors for activation [9], indicating that some B cells in the ectopic germinal centers are no longer subject to regulation by T cells. On the other hand, involvement of T cells in myasthenia gravis has been seen in thymoma. It has been reported that 55% of thymoma patients have myasthenia gravis [10]. Thymomas are tumors of the thymic epithelial origin. Thymic stromal cells (especially thymic epithelial cells) play a central role in training and selection of T cells. It is very likely that abnormal epithelial cells in thymoma patients provide aberrant signals to developing T cells, leading to abnormal training and selection of T cells and subsequent release of autoreactive helper T cells or defective regulatory T cells to the circulation. For example, regulatory T cells from myasthenia gravis patients have severely reduced suppressive function in a co-culture assay [11,12]. A similar defect has also been seen in other conventional T cells [13].
Genetic contribution to myasthenia gravis
What makes a patient more likely develop autoantibody against own proteins? Genetics clearly plays a role in myasthenia gravis. Twin studies indicate that the concordance rate for myasthenia gravis in monozygotic twins is 30-40% compare with 4-5% in dizygotic twins [14], supporting the role of genetic contribution to the disorder.
The HLA alleles have been implicated in several autoimmune disorders including myasthenia gravis. For example, HLA A1- B8-DR3-DQ2 haplotype has been associated with early onset myasthenia gravis in a Caucasian population [15-17]. The HLA DQ9 haplotype increases the risk of early onset myasthenia gravis in Southern Han Chinese while DRB1(*)09 allele is implicated in late onset myasthenia gravis in Northern Han Chinese [18,19]. DRB1*15:01, DQB1*05:02 and DRB1*16 have been linked to late onset myasthenia gravis in Norwegian and Italian groups and DQ5 allele to the disorder of the MuSK type in Southern and Northern European groups [20-23]. Within the HLA locus, a variant of the TNF gene is strongly linked to HLA A1-B8-DR3-DQ2 haplotype. A single nucleotide polymorphism (SNP), rs1800629, located at the position -308 within the TNF- α gene corresponds to two alleles: -308 A and -308 G. It has been shown that the TNF- α transcript level in the A allele is 2 fold of that in the G allele [24]. Not surprisingly, the A allele increases the risk of myasthenia gravis [25-27]. Another SNP, rs2233290, is located within the TNFAIP3-interacting protein 1 (TNIP1) gene. Normally TNIP1 inhibits signal transduction by cytokine receptors and nuclear receptors [28]. The SNP also has two alleles. One allele that yields Ala at the amino acid 151 is associated with early onset myasthenia gravis in a northern European population [29].
Some non-HLA loci have also been implicated in myasthenia gravis. For example, variants of ENOX1, PTPN22, CTLA4, FOXP3 and AChR-related genes are associated with myasthenia gravis. A variant of ENOX1 was linked to early onset myasthenia gravis in an Italian American kindred with parental consanguinity where 5 out of 10 siblings are affected [30]. ENOX1 encodes ecto-NADH oxidase. In those affected, the ENOX1 mRNA levels were significantly reduced [30]. PTPN22 encodes protein tyrosine phosphatase non-receptor 22, an intracellular protein tyrosine phosphatase. It binds C-terminal Src kinase (CSK). Both PTPN22 and CSK function together in mediating T cell activation [31]. In a PTPN22 variant, Arg at position 620 is replaced by Trp. The W620 variant binds CSK less efficiently than the R620 allele, leading to hyperactivation of CSK [32,33]. As a result, the W620 variant is prone to autoimmune diseases [34-37]. CTLA4 encodes a membrane-bound receptor on T cells. It represses IL-2, interferon-γ and IL-4 and CD86, ligands expressed in antigen-presenting cells [38]. CTLA4 is essential for development of regulatory T cells. SNPs located in the promoter region of the CTLA4 gene are linked to myasthenia gravis [39,40]. Likely, reduced expression level or the activity of CTLA4 is responsible for defects in regulatory T cells, leading to production of autoantibodies. FOXP3 encodes a transcription factor and it is a master regulator of regulatory T cell development and function [38]. Interestingly, a particular allele of the FOXP3 gene, FOXP3 IVS9+459G, reduces the risk of myasthenia gravis in a Han Chinese population [41]. It is suggested that this allele affects both the number and function of regulatory T cells, which confers beneficial effects [41].
Some genes coding for AChR are also implicated in myasthenia gravis. Different from loci described above, effects of AChR related genes on autoimmune responses are more specific to neuromuscular junctions and therefore specific to myasthenia gravis. In particular, polymorphism within the two AChR genes (CHRNA1 and CHRND) increases the risk of myasthenia gravis. CHRNA1 and CHRND encode the α- and δ- subunit of AChR, respectively. One of the CHRNA1 SNPs, rs16862847, is located within the promoter region of CHRNA1. A particular allele of this SNP is found to be twice more frequent in myasthenia gravis patients [42]. Further analyses show that the allele disrupts the binding site of a transcription factor [42].
Finally, sex hormones also play a role in several autoimmune diseases including myasthenia gravis. For example, among myasthenia gravis patients younger than 50 year old, 60-70% are females [43]. Many genes within the HLA locus contain estrogen response elements, suggesting that the genes related to immune responses are potentially controlled by estrogen [44]. In a mouse model of myasthenia gravis, estrogen enhances production of anti-AChR antibodies [45].
Environmental and epigenetic contribution to myasthenia gravis
The fact that the concordance rate for myasthenia gravis in monozygotic twins is less than 100% as described above indicates that environmental factors also contribute to the disorder. What environmental factors are involved and how they play a role in autoimmune responses are currently two key questions for active research. Although some evidence suggests that certain drugs such as D-penicillamine and interferon-β (IFN-β), and pollutants increase the risk of developing autoimmune diseases [46], main environmental risk factors for myasthenia gravis known to date are viruses and gut microbes.
An antiviral signature has been observed in myasthenia gravis thymus. For example, IFN-β, Toll-like receptor 4 (TLR4) and proteins involved in double-stranded RNA (dsRNA) signaling are over-expressed in the diseased thymus [47,48]. The dsRNA signaling pathway includes TLR3, protein kinase R, interferon regulatory factor 5 (IRF5) and IRF7 [48]. In addition, Epstein-Barr virus has been found in the myasthenia gravis thymus [46]. It is known that Epstein-Barr virus produces small RNA, which activates TLR3 signaling [49]. Further, when a synthetic dsRNA was injected into mice, the number of B cells was increased in the thymus and the anti-AChR antibody was found in the periphery [48]. These observations support the notion that virus plays a role in the pathogenesis of myasthenia gravis.
Microbes in the gastro-intestinal tract may also contribute to myasthenia gravis. In an animal model, differences in microbiota composition due to differences in hormones confer protection against type 1 diabetes in one gender but not in the other [50]. Since disturbance in the gut microbiota is known to cause autoimmune responses [51], it is hypothesized that the gut microbes also play a role in myasthenia gravis. Clearly more studies are needed to test this hypothesis.
How do these environmental factors affect host cell immune responses? There are potentially many ways in which environment affects host cell immunity. One emerging paradigm involves epigenetics. Mechanisms underlying epigenetics utilize DNA methylation, histone modification and microRNA to control gene expression without modifying genomic DNA sequence. Several microRNAs are associated with autoimmune diseases. For example, miR-146a, miR-155 and miR-326 promote T-cell response and inflammation while miR-145, miR-320a and let-7c have an opposite effect [52-56]. Either an elevated level in miR-146a, miR-155 or miR-326 or a reduced level in miR-320a, let-7c or miR-145 has been found in myasthenia gravis in humans or in an animal model of the disease [52,55,57]. Some targets of these microRNAs have been identified. For example, let-7c represses translation of IL-10 mRNA by targeting 3’-UTR of IL-10 at least in some cell lines [56]. Since many viruses produce dsRNA, dsRNA may provide a link between environment and genetics. In this paradigm, environmental factors such as viruses or drugs interfere with the levels of microRNAs. The aberrant pool of microRNAs in turn disturbs the balance of the immune system. Individuals with a particular genetic background may enhance the immune response, leading to a full blown autoimmune disease.
Diagnosis and treatment of myasthenia gravis
Diagnosis: Typical signs of myasthenia gravis include a drooping of the upper eyelid (ptosis), inability to hold heads straight, double vision (diplopia), difficulties in speaking, swallowing or chewing and difficulties in raising the arms or breathing. Occasionally, patients with myasthenia gravis may encounter alternating ptosis from one eye to the other and wrist drop [57].
It is important to distinguish myasthenia gravis from congenital myasthenic syndrome and Lambert-Eaton myasthenic syndrome. Congenital myasthenic syndrome is an inherited neuromuscular disorder caused by genetic defects that affect proteins in the neuromuscular junction [58]. As a result, myasthenic syndrome is not an autoimmune disorder. On the other hand, Lambert–Eaton myasthenic syndrome (or Eaton–Lambert syndrome) is an autoimmune disorder characterized by muscle weakness of the limbs. However, different from myasthenia gravis, Eaton–Lambert syndrome is caused by autoantibodies against presynaptic membrane proteins, especially voltage-gated calcium channel [59]. The key sign of myasthenia gravis is muscle weakness that improves with rest.
Two tests can be performed to assist diagnosis of myasthenia gravis: Ice-pack test and edrophonium test. The ice-pack test is carried out by placing a small ice bag over the drooping eyelids for 2 to 5 minutes. If the test is positive, cooling often ameliorates the characteristic ptosis symptom [60]. Evidence suggests that by cooling the tissue, the acetylcholinesterase is less active [61]. Edrophonium test involves injection of edrophonium chloride (Tensilon) intravenously. Edrophonium chloride blocks acetylcholinesterase activities [62]. If injection of edrophonium chloride results in a sudden, although temporary, improvement in the muscle strength, this will support the diagnosis of myasthenia gravis.
To confirm the diagnosis of myasthenia gravis, a blood test should be performed. A laboratory test for anti-AChR, anti- MuSK and anti-LRP4 antibodies should be included. In 85 to 90% of patients with generalized myasthenia gravis, anti-AChR antibodies are detected in the serum [43]. A small group of patients have either anti-MuSK (~ 4%) or anti-LRP4 (~2%) antibodies. In the rest of patients, these antibodies have not been detected so far and, therefore, they are classified into a seronegative group.
For patients in the seronegative group, electrophysiological tests can be useful in diagnosis.
Two tests currently are recommended: Repetitive nerve stimulation test and single-fiber electromyography.
Repetitive nerve stimulation test involves electrical stimulation delivered to a motor nerve repeatedly several times per second and recording of the muscle electrical response. The test is positive if the muscle electrical response falls below threshold by more than 10%.
Single-fiber electromyography, a more sensitive test than repetitive nerve stimulation, involves measuring the temporal variability in their firing patterns of two muscle fibers belonging to the same motor unit. Two abnormal firing patterns, “jitter” and “blocking”, are diagnostic. Jitter describes the abnormal variation in the time interval between action potentials of adjacent muscle fibers while blocking refers to the failure of nerve impulses to elicit action potentials in adjacent muscle fibers of the same motor unit. Jitter is seen in over 85% of patients with the ocular form and more than 90% of patients with the generalized form of myasthenia gravis [63].
To test whether autoantibody production is due to any abnormality in the thymus (e.g., thymoma), chest computed tomography (CT) or magnetic resonance imaging (MRI) is recommended [64].
Treatment: Current therapy for myasthenia gravis includes acetylcholinesterase (AchE) inhibitors, immunomodulating agents, intravenous immune globulin (IVIg), plasmapheresis and thymectomy.
Acetylcholinesterase inhibitors: Pyridostigmine and neostigmine are the first line medications to treat mild cases of myasthenia gravis [65]. Both of them are reversible competitive inhibitors of acetylcholinesterase at the neuromuscular junction.
Immunomodulating agents: This group of therapy includes glucocorticoids (e.g., prednisone), cyclophosphamide, azathioprine, mycophenolate mofetil, methotrexate, rituximab and cyclosporine (or ciclosporin). These drugs alone or in combination can be used to treat difficult cases of myasthenia gravis. Glucocorticoids suppress expression of IL-2 along with a few other cytokines, whereby it reduces B- and T-cell proliferation [66]. In the meantime, glucocorticoids also increase expression of lipocortin-1, which reduces production of prostaglandins by inhibiting phospholipase A2 activity [67]. Glucocorticoids can be effective in relieving the condition for a short-term. Cyclophosphamide is an alkylating agent. Azathioprine is a purine analog. Mycophenolate mofetil is a non-competitive inhibitor of inosine-5’-monophosphate dehydrogenase, a key enzyme in the de novo guanosine nucleotide synthesis. Methotrexate is a folic acid analog. A common mode of action among cyclophosphamide, azathioprine, mycophenolate mofetil and methotrexate is to inhibit DNA synthesis, whereby they inhibit proliferation of both T- and B-cells. Rituximab is a monoclonal antibody against B-cell marker CD20 [68]. Binding of CD20 by rituximab leads to death of the lympocytes [68]. Ciclosporin is a calcineurin inhibitor that reduces expression of the pro-inflammatory factor interleukin-2 (IL-2). Taken together, all of these immunomodulating agents either block proliferation of lymphocytes and/or reduce production of pro-inflammatory factors.
Intravenous immunoglobulin (IVIg): For patients with severe myasthenia gravis, intravenous immunoglobulin may be considered. It involves delivery of immunoglobulin (IVIg) to patients via an intravenous route. IVIgs are sterile IgG products purified from pooled human plasma and typically contain more than 95% unmodified IgG. The high content of antiidiotypes against autoantibodies in IVIg facilitates its ability to neutralize autoantibodies, as is demonstrated in patients with acquired hemophilia that is caused by autoantibodies against factor VIII [69].
Plasmapheresis: This therapy involves removal, treatment and return of plasma. The beneficial effect can be seen within the first week of the treatment. However, the improvement usually does not last more than two months. Plasmapheresis can be used for severe or rapidly worsening cases of myasthenia gravis [70].
Thymectomy: Surgical removal of the thymus is recommended for patients with thyomoma and for patients aged at 10-55 years with generalized myasthenia gravis [71].
Future prospect
To date, the genome-wide association studies (GWAS) have provided several hundreds of genetic loci associated with some common complex disorders [72]. However, most variants identified so far only contribute to a small increment in risk, leaving a gap in understanding between genetic variants and the phenotypic variations. Deeper understanding of the interplay between environment and epigenetics may help us eventually close the gap. Myasthenia gravis is one of the wellcharacterized complex disorders. Recent work has raised the possibility that antiviral responses trigger production of autoantibodies and dsRNA may provide a link between viruses and host immunity in myasthenia gravis. Other aspects of epigenetics are known to play a role in autoimmune disorders. For example, a histone deacetylase inhibitor (HDI) improves juvenile arthritis in a clinical trial [73]. HDIs are also effective in treatment of rheumatoid arthritis, lupus and type 1 diabetes in experimental settings [53]. It remains to be tested whether environment through DNA methylation and histone acetylation triggers autoimmune responses in myasthenia gravis.
Current understanding of myasthenia gravis has prompted the development of some potential therapies for the disorder (Table 1).
| Treatment strategy | Drugs | Targets | Mechansim |
| Inhibition of complement | anti-C5 antibody | Complement | Blocking complement |
| Soluble CR1 | Complement | Competitive inhibitor of complement |
|
| Epigenetic modification | HDIs | HDACs | increasing gene expression |
| DNMT inhibitors | DNMT | Altering DNA methylation | |
| Modification of APCs | APCs treated with cytokine | T cells | APCs treated with IL-10, IFN-g and TGF-β suppress T cell autoimmunity |
| T cell vaccination | Synthetic peptides | CD4+ T cells | inducing antibodies that block binding of T cells to autoantigens |
| Elimination of B cells | AChR-toxin conjugate | B cells | Toxin elicits death signals to B cells |
| Elimination of T cells | APCs expressing FasL | T cells | FasL elicits death signals to T cells |
APCs, antigen-presenting cells; FasL, Fas ligand
Table 1: Future therapeutics for myasthenia gravis.
1) Complement inhibitors: Binding of autoantibodies to autoantigens will activate complement pathways, leading to destruction of target cells (e.g., muscle). Complement inhibitors having been tested in experimental settings include soluble complement receptors and anti-complement antibodies. For example, soluble complement receptor 1 and anti-C6 antibodies are effective in animal models of myasthenia gravis [74,75]. The efficacy of these in treating myasthenia gravis patients awaits clinical trials.
2) Epigenetic therapeutics: The most studied epigenetic therapeutics so far is HDIs. The HDIs promote acetylation of histone proteins, leading to an increase in gene expression (e.g., pro-apoptotic and anti-inflammatory genes). Originally developed as chemotherapy for cancers [76], they have been tested to treat some autoimmune disorders including rheumatoid arthritis, lupus, type 1 diabetes and juvenile arthritis as described above. The second class of epigenetic therapeutics includes inhibitors of DNA methyltransferase (DNMT). DNMT inhibitors interfere with DNA methylation and they are currently being tested for their efficacy in treating autoimmune disorder in animals [77]. Similar to complement inhibitors, HDIs and DNMT inhibitors are non-specific to myasthenia gravis.
3) Modified antigen presenting cells: Dendritic cells, after exposed to IFN-γ, IL-10 or TGF-β in vitro and injected into rats with myasthenia gravis, ameliorate the muscle condition [78-80]. Treated antigen presenting cells seem to reduce production of anti-AChR antibodies and therefore increase the antigen-specific tolerance. This approach is more specific to myasthenia gravis than complement inhibitors and epigenetic therapeutics. It remains to be tested in clinical trials.
4) T cell vaccination: One approach to T cell vac ination is to utilize anti-T cell receptor antibodies that recognize the antigen-binding sites of T cell receptor. The antibodies interfere with binding between autoantibodies and autoantigens. The anti-T cell receptor antibodies can be produced in vitro and administered through injection or they could be induced in vivo using synthetic peptides [81,82]. This has been used in clinical trials for several autoimmune disorders including multiple sclerosis, rheumatoid arthritis and psoriasis [81]. Second approach to T cell vaccination is to use synthetic peptides that are variants of epitopes recognized by pathogenic T cells. The peptides can bind both the T cell receptors and the MHC II molecules but can’t stimulate the T cells. As a result, synthetic peptides compete with endogenous self-antigens for binding to T cells, whereby they turn off the autoimmune responses. T cell vaccination is a specific approach to myasthenia gravis but its efficacy in vivo awaits clinical trials.
5) Elimination of B cells: When the AChR protein conjugated with a toxin is administered to rats with myasthenia gravis, the conjugate elicits cellular toxicity, leading to elimination of B cells [83]. This approach is specific to myasthenia gravis. However, the side effect has to be carefully monitored during clinical trials since the toxin may be toxic to other cells as well.
6) Elimination of T cells: CD4+ T cells that recognize the autoantigen AChR can be eliminated by Fas-mediated apoptosis. This approach utilizes antigen presenting cells that express three proteins: AChR, Fas ligand (FasL) and truncated FADD [84]. FasL triggers the death signal upon the target T cells while truncated FADD protects the antigen presenting cells from self-destruction. This approach is effective in cultured cells and specific to myasthenia gravis [84]. However, its efficacy needs to be tested in animals first.
Conclusion
Myasthenia gravis is perhaps the best characterized autoimmune disorder. Fine mapping of molecular components of signal pathways at neuromuscular junctions and simplicity of the symptom have all made this disorder a powerful model for understanding pathogenesis of autoimmune disorders. Unravelling the link between environment and epigenetics may provide a key to further understanding the causes of myasthenia gravis and a new rationale for designing specific therapeutics for the autoimmune disease.
Acknowledgement
We thank the Research Committee of Saint James School of Medicine for support of this work and the related molecular biology research.
Conflicts of Interest
The authors declare that there is no conflict of interests.
References
- Hughes T (2005) The early history of myasthenia gravis. NeuromusculDisord 15:878-886.
- Meriggioli MN, Sanders DB (2009) Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 8:475-490.
- Le Panse R, Berrih-Aknin S (2013) Autoimmune myasthenia gravis: autoantibody mechanisms and new developments on immune regulation. CurrOpinNeurol 26:569-576.
- Sandow A (1965) Excitation-contraction coupling in skeletal muscle. Pharmacol Rev 17:265-320.
- Sine SM (2012) End-plate acetylcholine receptor: structure, mechanism, pharmacology, and disease. Physiol Rev 92:1189-1234.
- Wu H, Xiong WC, Mei L (2010) To build a synapse: signaling pathways in neuromuscular junction assembly. Development 137:1017-1033.
- Kondo M (2010) Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol Rev 238:37-46.
- Berrih S, Morel E, Gaud C, Raimond F, Le Brigand H, et al. (1984) Anti-AChR antibodies, thymic histology, and T cell subsets in myasthenia gravis. Neurology 34:66-71.
- Leprince C, Cohen-Kaminsky S, Berrih-Aknin S, Vernet-Der Garabedian B, Treton D, et al. (1990) Thymic B cells from myasthenia gravis patients are activated B cells. Phenotypic and functional analysis. J Immunol 145:2115-2122.
- Filosso PL, Galassi C, Ruffini E, Margaritora S, Bertolaccini L, et al. (2013) Thymoma and the increased risk of developing extrathymic malignancies: a multicentre study. Eur J CardiothoracSurg 44:219-224.
- Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S (2005) Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood 105:735-741.
- Zhang Y, Wang HB, Chi LJ, Wang WZ (2009) The role of FoxP3+CD4+CD25hi Tregs in the pathogenesis of myasthenia gravis. ImmunolLett 122:52-57.
- Gradolatto A, Nazzal D, Truffault F, Bismuth J, Fadel E, et al. (2014) Both Treg cells and Tconv cells are defective in the Myasthenia gravis thymus: roles of IL-17 and TNF-alpha. J Autoimmun 52:53-63.
- Ramanujam R, Pirskanen R, Ramanujam S,Hammarstrom L (2011) Utilizing twins concordance rates to infer the predisposition to myasthenia gravis. Twin Res Hum Genet 14:129-136.
- Giraud M, Beaurain G, Eymard B, Tranchant C, Gajdos P, et al. (2004) Genetic control of autoantibody expression in autoimmune myasthenia gravis: role of the self-antigen and of HLA-linked loci. Genes Immun 5:398-404.
- Janer M, Cowland A, Picard J, Campbell D, Pontarotti P, et al. (1999) A susceptibility region for myasthenia gravis extending into the HLA-class I sector telomeric to HLA-C. Hum Immunol 60:909-917.
- Vandiedonck C, Beaurain G, Giraud M, Hue-Beauvais C, Eymard B, Tranchant C, Gajdos P, Dausset J and Garchon HJ (2004) Pleiotropic effects of the 8.1 HLA haplotype in patients with autoimmune myasthenia gravis and thymus hyperplasia. ProcNatlAcadSci U S A 101:15464-15469.
- Xie YC, Qu Y, Sun L, Li HF, Zhang H, Shi HJ, Jiang B, Zhao Y, Qiao SS, Wang SH and Wang DX (2011) Association between HLA-DRB1 and myasthenia gravis in a northern Han Chinese population. J ClinNeurosci 18:1524-1527.
- Zhu WH, Lu JH, Lin J, Xi JY, Lu J, Luo SS, Qiao K, Xiao BG, Lu CZ and Zhao CB (2012) HLA-DQA1*03:02/DQB1*03:03:02 is strongly associated with susceptibility to childhood-onset ocular myasthenia gravis in Southern Han Chinese. J Neuroimmunol 247:81-85.
- Bartoccioni E, Scuderi F, Augugliaro A, ChiatamoneRanieri S, Sauchelli D, Alboino P, Marino M and Evoli A (2009) HLA class II allele analysis in MuSK-positive myasthenia gravis suggests a role for DQ5. Neurology 72:195-197.
- Maniaol AH, Elsais A, Lorentzen AR, Owe JF, Viken MK, Saether H, Flam ST, Brathen G, Kampman MT, Midgard R, Christensen M, Rognerud A, Kerty E, Gilhus NE, Tallaksen CM, Lie BA and Harbo HF (2012) Late onset myasthenia gravis is associated with HLA DRB1*15:01 in the Norwegian population. PLoS One 7:e36603.
- Niks EH, Kuks JB, Roep BO, Haasnoot GW, Verduijn W, Ballieux BE, De Baets MH, Vincent A and Verschuuren JJ (2006) Strong association of MuSK antibody-positive myasthenia gravis and HLA-DR14-DQ5. Neurology 66:1772-1774.
- Testi M, Terracciano C, Guagnano A, Testa G, Marfia GA, Pompeo E, Andreani M and Massa R (2012) Association of HLA-DQB1 *05:02 and DRB1 *16 Alleles with Late-Onset, Nonthymomatous, AChR-Ab-Positive Myasthenia Gravis. Autoimmune Dis 2012:541760.
- Kroeger KM, Carville KS and Abraham LJ (1997) The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. MolImmunol 34:391-399.
- Huang DR, Pirskanen R, Matell G and Lefvert AK (1999) Tumour necrosis factor-alpha polymorphism and secretion in myasthenia gravis. J Neuroimmunol 94:165-171.
- Skeie GO, Pandey JP, Aarli JA and Gilhus NE (1999) TNFA and TNFB polymorphisms in myasthenia gravis. Arch Neurol 56:457-461.
- Zelano G, Lino MM, Evoli A, Settesoldi D, Batocchi AP, Torrente I and Tonali PA (1998) Tumour necrosis factor beta gene polymorphisms in myasthenia gravis. Eur J Immunogenet 25:403-408.
- Zhang S, Fukushi M, Hashimoto S, Gao C, Huang L, Fukuyo Y, Nakajima T, Amagasa T, Enomoto S, Koike K, Miura O, Yamamoto N and Tsuchida N (2002) A new ERK2 binding protein, Naf1, attenuates the EGF/ERK2 nuclear signaling. BiochemBiophys Res Commun 297:17-23.
- Gregersen PK, Kosoy R, Lee AT, Lamb J, Sussman J, McKee D, Simpfendorfer KR, Pirskanen-Matell R, Piehl F, Pan-Hammarstrom Q, Verschuuren JJ, Titulaer MJ, Niks EH, Marx A, Strobel P, Tackenberg B, Putz M, Maniaol A, Elsais A, Tallaksen C, Harbo HF, Lie BA, Raychaudhuri S, de Bakker PI, Melms A, Garchon HJ, Willcox N, Hammarstrom L and Seldin MF (2012) Risk for myasthenia gravis maps to a (151) Pro-->Ala change in TNIP1 and to human leukocyte antigen-B*08. Ann Neurol 72:927-935.
- Landoure G, Knight MA, Stanescu H, Taye AA, Shi Y, Diallo O, Johnson JO, Hernandez D, Traynor BJ, Biesecker LG, Center NIHIS, Elkahloun A, Rinaldi C, Vincent A, Willcox N, Kleta R, Fischbeck KH and Burnett BG (2012) A candidate gene for autoimmune myasthenia gravis. Neurology 79:342-347.
- Vang T, Miletic AV, Bottini N and Mustelin T (2007) Protein tyrosine phosphatase PTPN22 in human autoimmunity. Autoimmunity 40:453-461.
- Lefvert AK, Zhao Y, Ramanujam R, Yu S, Pirskanen R and Hammarstrom L (2008) PTPN22 R620W promotes production of anti-AChR autoantibodies and IL-2 in myasthenia gravis. J Neuroimmunol 197:110-113.
- Vandiedonck C, Capdevielle C, Giraud M, Krumeich S, Jais JP, Eymard B, Tranchant C, Gajdos P and Garchon HJ (2006) Association of the PTPN22*R620W polymorphism with autoimmune myasthenia gravis. Ann Neurol 59:404-407.
- Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, Conn MT, Chang M, Chang SY, Saiki RK, Catanese JJ, Leong DU, Garcia VE, McAllister LB, Jeffery DA, Lee AT, Batliwalla F, Remmers E, Criswell LA, Seldin MF, Kastner DL, Amos CI, Sninsky JJ and Gregersen PK (2004) A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet 75:330-337.
- Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D and Mustelin T (2004) A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 36:337-338.
- Greve B, Hoffmann P, Illes Z, Rozsa C, Berger K, Weissert R and Melms A (2009) The autoimmunity-related polymorphism PTPN22 1858C/T is associated with anti-titin antibody-positive myasthenia gravis. Hum Immunol 70:540-542.
- Lee YH, Rho YH, Choi SJ, Ji JD, Song GG, Nath SK and Harley JB (2007) The PTPN22 C1858T functional polymorphism and autoimmune diseases--a meta-analysis. Rheumatology (Oxford) 46:49-56.
- Sakaguchi S, Yamaguchi T, Nomura T and Ono M (2008) Regulatory T cells and immune tolerance. Cell 133:775-787.
- Gu M, Kakoulidou M, Giscombe R, Pirskanen R, Lefvert AK, Klareskog L and Wang X (2008) Identification of CTLA-4 isoforms produced by alternative splicing and their association with myasthenia gravis. ClinImmunol 128:374-381.
- Wang XB, Pirskanen R, Giscombe R and Lefvert AK (2008) Two SNPs in the promoter region of the CTLA-4 gene affect binding of transcription factors and are associated with human myasthenia gravis. J Intern Med 263:61-69.
- Zhang J, Chen Y, Jia G, Chen X, Lu J, Yang H, Zhou W, Xiao B, Zhang N and Li J (2013) FOXP3 -3279 and IVS9+459 polymorphisms are associated with genetic susceptibility to myasthenia gravis. NeurosciLett 534:274-278.
- Giraud M, Taubert R, Vandiedonck C, Ke X, Levi-Strauss M, Pagani F, Baralle FE, Eymard B, Tranchant C, Gajdos P, Vincent A, Willcox N, Beeson D, Kyewski B and Garchon HJ (2007) An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature 448:934-937.
- Berrih-Aknin S and Le Panse R (2014) Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun 52:90-100.
- Kaur M, Schmeier S, MacPherson CR, Hofmann O, Hide WA, Taylor S, Willcox N and Bajic VB (2008) Prioritizing genes of potential relevance to diseases affected by sex hormones: an example of myasthenia gravis. BMC Genomics 9:481.
- Delpy L, Douin-Echinard V, Garidou L, Bruand C, Saoudi A and Guery JC (2005) Estrogen enhances susceptibility to experimental autoimmune myasthenia gravis by promoting type 1-polarized immune responses. J Immunol 175:5050-5057.
- Cavalcante P, Cufi P, Mantegazza R, Berrih-Aknin S, Bernasconi P and Le Panse R (2013) Etiology of myasthenia gravis: innate immunity signature in pathological thymus. Autoimmun Rev 12:863-874.
- Bernasconi P, Barberis M, Baggi F, Passerini L, Cannone M, Arnoldi E, Novellino L, Cornelio F and Mantegazza R (2005) Increased toll-like receptor 4 expression in thymus of myasthenic patients with thymitis and thymic involution. Am J Pathol 167:129-139.
- Cufi P, Dragin N, Weiss JM, Martinez-Martinez P, De Baets MH, Roussin R, Fadel E, Berrih-Aknin S and Le Panse R (2013) Implication of double-stranded RNA signaling in the etiology of autoimmune myasthenia gravis. Ann Neurol 73:281-293.
- Iwakiri D, Zhou L, Samanta M, Matsumoto M, Ebihara T, Seya T, Imai S, Fujieda M, Kawa K and Takada K (2009) Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J Exp Med 206:2091-2099.
- Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y and Chervonsky AV (2013) Gender bias in autoimmunity is influenced by microbiota. Immunity 39:400-412.
- Sathyabama S, Khan N and Agrewala JN (2014) Friendly pathogens: prevent or provoke autoimmunity. Crit Rev Microbiol 40:273-280.
- Cheng Z, Qiu S, Jiang L, Zhang A, Bao W, Liu P and Liu J (2013) MiR-320a is downregulated in patients with myasthenia gravis and modulates inflammatory cytokines production by targeting mitogen-activated protein kinase 1. J ClinImmunol 33:567-576.
- De Santis M and Selmi C (2012) The therapeutic potential of epigenetics in autoimmune diseases. Clin Rev Allergy Immunol 42:92-101.
- Hedrich CM and Tsokos GC (2011) Epigenetic mechanisms in systemic lupus erythematosus and other autoimmune diseases. Trends Mol Med 17:714-724.
- Jiang L, Cheng Z, Qiu S, Que Z, Bao W, Jiang C, Zou F, Liu P and Liu J (2012) Altered let-7 expression in Myasthenia gravis and let-7c mediated regulation of IL-10 by directly targeting IL-10 in Jurkat cells. IntImmunopharmacol 14:217-223.
- Wang J, Zheng S, Xin N, Dou C, Fu L, Zhang X, Chen J, Zhang Y, Geng D, Xiao C, Cui G, Shen X, Lu Y, Wang J, Dong R, Qiao Y and Zhang Y (2013) Identification of novel MicroRNA signatures linked to experimental autoimmune myasthenia gravis pathogenesis: down-regulated miR-145 promotes pathogenetic Th17 cell response. J NeuroimmunePharmacol 8:1287-1302.
- Keesey JC (2004) Clinical evaluation and management of myasthenia gravis. Muscle Nerve 29:484-505.
- Engel AG, Shen XM, Selcen D and Sine SM (2015) Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 14:420-434.
- Hulsbrink R and Hashemolhosseini S (2014) Lambert-Eaton myasthenic syndrome - diagnosis, pathogenesis and therapy. ClinNeurophysiol 125:2328-2336.
- Kearsey C, Fernando P, D'Costa D and Ferdinand P (2010) The use of the ice pack test in myasthenia gravis. JRSM Short Rep 1:14.
- Ricker K, Hertel G and Stodieck S (1977) Influence of temperature on neuromuscular transmission in myasthenia gravis. J Neurol 216:273-282.
- Osserman KE and Kaplan LI (1952) Rapid diagnostic test for myasthenia gravis: increased muscle strength, without fasciculations, after intravenous administration of edrophonium (tensilon) chloride. J Am Med Assoc 150:265-268.
- Oh SJ, Kim DE, Kuruoglu R, Bradley RJ and Dwyer D (1992) Diagnostic sensitivity of the laboratory tests in myasthenia gravis. Muscle Nerve 15:720-724.
- Li Y, Arora Y and Levin K (2013) Myasthenia gravis: newer therapies offer sustained improvement. Cleve Clin J Med 80:711-721.
- Gilhus NE and Verschuuren JJ (2015) Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 14:1023-1036.
- Leung DY and Bloom JW (2003) Update on glucocorticoid action and resistance. J Allergy ClinImmunol 111:3-22; quiz 23.
- Goppelt-Struebe M, Wolter D and Resch K (1989) Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br J Pharmacol 98:1287-1295.
- Weiner GJ (2010) Rituximab: mechanism of action. SeminHematol 47:115-123.
- Kazatchkine MD and Kaveri SV (2001) Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 345:747-755.
- Gold R and Schneider-Gold C (2008) Current and future standards in treatment of myasthenia gravis. Neurotherapeutics 5:535-541.
- Sussman J, Farrugia ME, Maddison P, Hill M, Leite MI and Hilton-Jones D (2015) Myasthenia gravis: Association of British Neurologists' management guidelines. PractNeurol 15:199-206.
- Naidoo N and Chia KS (2009) Discovering gene-environment interactions in the post-genomic era. J Prev Med Public Health 42:356-359.
- Vojinovic J, Damjanov N, D'Urzo C, Furlan A, Susic G, Pasic S, Iagaru N, Stefan M and Dinarello CA (2011) Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 63:1452-1458.
- Biesecker G and Gomez CM (1989) Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J Immunol 142:2654-2659.
- Piddlesden SJ, Jiang S, Levin JL, Vincent A and Morgan BP (1996) Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J Neuroimmunol 71:173-177.
- Chen S and Sang N (2011) Histone deacetylase inhibitors: the epigenetic therapeutics that repress hypoxia-inducible factors. J Biomed Biotechnol 2011:197946.
- Baer-Dubowska W, Majchrzak-Celinska A and Cichocki M (2011) Pharmocoepigenetics: a new approach to predicting individual drug responses and targeting new drugs. Pharmacol Rep 63:293-304.
- Adikari SB, Lian H, Link H, Huang YM and Xiao BG (2004) Interferon-gamma-modified dendritic cells suppress B cell function and ameliorate the development of experimental autoimmune myasthenia gravis. ClinExpImmunol 138:230-236.
- Duan RS, Adikari SB, Huang YM, Link H and Xiao BG (2004) Protective potential of experimental autoimmune myasthenia gravis in Lewis rats by IL-10-modified dendritic cells. Neurobiol Dis 16:461-467.
- Yarilin D, Duan R, Huang YM and Xiao BG (2002) Dendritic cells exposed in vitro to TGF-beta1 ameliorate experimental autoimmune myasthenia gravis. ClinExpImmunol 127:214-219.
- Cohen-Kaminsky S and Jambou F (2005) Prospects for a T-cell receptor vaccination against myasthenia gravis. Expert Rev Vaccines 4:473-492.
- Dayan M, Sthoeger Z, Neiman A, Abarbanel J, Sela M and Mozes E (2004) Immunomodulation by a dual altered peptide ligand of autoreactive responses to the acetylcholine receptor of peripheral blood lymphocytes of patients with myasthenia gravis. Hum Immunol 65:571-577.
- Urbatsch IL, Sterz RK, Peper K and Trommer WE (1993) Antigen-specific therapy of experimental myasthenia gravis with acetylcholine receptor-gelonin conjugates in vivo. Eur J Immunol 23:776-779.
- Wu JM, Wu B, Miagkov A, Adams RN and Drachman DB (2001) Specific immunotherapy of experimental myasthenia gravis in vitro: the "guided missile" strategy. Cell Immunol 208:137-147
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences