Role of Bromelain as Herbal Anti-Inflammatory Compound Using In Vitro and In Vivo Model of Colitis
Nirmal Verma#, Naresh Kumar Meena#, Ishani Majumdar and Jaishree Paul*
Nirmal Verma#, Naresh Kumar Meena#, Ishani Majumdar and Jaishree Paul*
School of Life Sciences, Lab 441, Jawaharlal Nehru University, New Delhi, India
- *Corresponding Author:
- Dr. Jaishree Paul
Ph.D., School of Life Sciences, Lab 441
Jawaharlal Nehru University, New Delhi-110067, India
E-mail: jaishree.paul@gmail.com
Received date: December 09, 2017; Accepted date: December 28, 2017; Published date: December 30, 2017
Citation: Verma N, Meena NK, Majumdar I, Paul J (2017) Role of Bromelain as Herbal Anti-Inflammatory Compound Using In Vitro and In Vivo Model of Colitis. J Autoimmune Disord Vol 3:52.
Copyright: © 2017 Verma N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Bromelain is a well known anti-inflammatory compound derived from pineapple fruit and stem extract. It has been shown previously that bromelain decreases colonic inflammation in mice models of inflammatory disease. However the mechanism by which anti-inflammatory effect of bromelain is mediated, is unknown. In this study, we evaluated the effect of bromelain on intestinal inflammation using Lipopolysaccharide (LPS) treated human intestinal adenocarcinoma cell line (HT29 cells) as in vitro model system and Dextran Sulfate Sodium (DSS) induced mouse model of colitis as in vivo system. The expression of TLR4, PPARγ (an antagonist of TLR4), pro-inflammatory cytokines like TNFα and IL8 were evaluated in the in vitro model while in DSS induced mice model, several parameters of inflammation were examined after bromelain treatment. HT29 cells were challenged with 100 ng/ml LPS for 24 h and then treated with 20 μg/ml of pure bromelain for 24 h. Real-time PCR was carried out for quantifying relative expression of TLR4, PPARγ, IL8 and TNFα. Experimental colitis was induced in Swiss albino mice by adding 2% DSS in their drinking water for seven days followed by one day with water. Mice were co-treated with bromelain (100 mg/kg of body weight/day and 200 mg/kg of body weight/day) which was administered through oral gavage. Our in-vitro results demonstrated that LPS induced TLR4 mRNA expression decreases after bromelain treatment while that of PPARγ mRNA increases. In addition, mRNA expression of pro-inflammatory cytokines IL8 and TNFα also decreased significantly after bromelain treatment in LPS challenged HT29 cells. On the other hand, parameters of inflammation were significantly improved in DSS induced colitis model of mice after bromelain treatment. These results show that bromelain possibly negatively modulates expression of TLR4 and restricts NFκB activation to ameliorate inflammation during colitis.
Keywords
Bromelain; Anti-inflammatory; PPARg; cytokine signaling
Introduction
Chronic inflammation is associated with altered cell signaling pathways resulting in increased levels of inflammatory markers and free radicals that cause cell damage and clinical symptoms of diseases like obesity, diabetes and inflammatory bowel diseases. Inflammatory bowel diseases arise from an inappropriate immune response towards commensal microbes in genetically susceptible individuals. Synthesis and release of different pro-inflammatory mediators are upregulated like reactive oxygen and nitrogen metabolites, and cytokines like TNF-α, IL-6 and IL-1 that initiate and perpetuate the inflammatory response in the gut [1-3].
Dextran Sulfate Sodium (DSS) induced murine models of intestinal inflammation are considered as an appropriate model because they are simple to induce and the onset, duration, and severity of inflammation can be appropriately monitored within a short span of time. DSS induced colitis are well-established animal models of mucosal inflammation that have been used for over 2 decades in the study of IBD pathogenesis and preclinical studies [4-7].
Bromelain is obtained from the crude extract of pineapple plant's (Ananas comosus) fruit and the stem consisting of different proteinases although its exact chemical composition is not entirely known [8-10]. Bromelain comprises of a group of sulfhydryl proteolytic enzymes and consists of various cysteine proteases. Bromelain activation is dependent on the constituent cysteine. Bromelain consists of a combination of several thiol endopeptidases apart from peroxidases, glucosidases, acid phosphatase, glycoproteins, cellulases, organically intact Ca2+ and carbohydrates. Bromelain has been used for medicinal purposes as anti-inflammatory, fibrinolytic agents, and for its anti-thrombotic properties and zero side effects. Bromelain also has good absorbance capability in GI tract (upto 40%) where most orally ingested enzyme is destroyed by gastric juices [9].
Oral supplementation with bromelain decreases inflammation of the colon in animals with active inflammatory bowel disease (IBD). Bromelain supplementation also significantly reduced inflammation in test animals with established IBD [11]. In vitro studies revealed that bromelain treatment of cultured inflamed tissue of UC and CD patients exhibited decrease secretion of G-CSF, granulocyte-macrophage-colony-stimulating factor (GM-CSF), IFN-gamma, CCL4/macrophage inhibitory protein (MIP)-1beta, and TNF when compared with non-IBD controls [12].
The contribution of TLR signaling in the pathogenesis of IBD has been established. Recent findings in diverse murine models of colitis have helped to reveal the mechanistic importance of TLR dysfunction in IBD pathogenesis [13,14]. The peroxisome proliferator-activated receptor gamma (PPARγ) is a nuclear receptor known to over express in fat tissues but also expressed in gut tissues and responsible for insulin resistance and inflammation. PPARγ is expressed mainly in the epithelial layer of gut mucosa. Recently it has also emerged as a factor responsible for colon cancer and gut inflammation. PPARγ expression has been found to decrease by 60% in gut of IBD patients both at transcript and protein level which shows its role in IBD pathology [15].
Further, it has been established that lipopolysaccharide (LPS), a TLR4 ligand, functions as an antagonist of PPARγ [16]. Using endotoxin-induced uveitis (EIU) as a model, it was demonstrated that TLR4 expression was negatively regulated by PPARγ. Further, it was also observed that activation of PPARγ ameliorates inflammation by inhibiting NFκB activity, suppressing pro-inflammatory cytokine production [17]. Role of this receptor in various diseases has also been reported [18]. These studies indicate the role of PPARγ as a potential therapeutic target.
In this study, we attempted to dissect out the possible anti-inflammatory signaling mechanism of bromelain in context to colitis using in vitro model to understand the effect of bromelain on PPARγ and TLR4 expression. Further, we examined whether the anti-inflammatory effects of bromelain can be observed in a DSS-induced mice model of colitis when introduced orally.
Methods and Materials
Animal cell culture
The human colorectal adeno carcinoma cell line HT29 (NCCS, Pune, India) was maintained in DMEM High Glucose media supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Invitrogen), penicillin (50 units/ml) and streptomycin (50 μg/ml) in a fully humidified atmosphere with 5% CO2 at 37°C. For cell stimulation, the cells were incubated with or without 100 ng/ml LPS in DMEM for the indicated periods (24 h) [19]. After LPS stimulation cells were treated with 20 μg/ml bromelain (B5144, Sigma Aldrich, St. Louis, USA) in triplicate for 24 h and total RNA was isolated from each treatment.
RNA extraction and Real-Time PCR
Total RNA from cells was extracted using Trizol reagent (Sigma Aldrich, Missouri, USA). The concentration of RNA was adjusted to 1 μg/μL with RNase free distilled water. Reverse transcription of total RNA was performed by means of the Revert Aid First Strand cDNA Synthesis kit (Fermentas, St. Leon Rot, Germany) using 1 μg of total RNA per sample in a final volume of 20 μl. The quality of cDNA was checked by normal PCR reactions and subjected to Real-time PCR in 7500 Real-time PCR system. SYBR green universal PCR master mix from Applied Biosystems (California, USA) was used as per the instructions of the supplier. Prior to each quantitative real-time PCR, the cDNA was diluted appropriately. For all experiments, RNA of untreated cells were isolated (control) at all the time points and the GAPDH normalized values (ÃÆâÃâÃâ Ãâââ¬Â CT) of the stimulated HT 29 cells were expressed relative to the normalized values of the respective control cells (ÃÆâÃâÃâ Ãâââ¬Â ÃÆâÃâÃâ Ãâââ¬Â CT). We carried out an independent experiment to show that GAPDH was not regulated by the applied mediators used in our study. The CT values of all genes ranged from 20 to 30. To quantify gene expression, the comparative threshold cycle method for relative quantification (2-ÃÆâÃâÃâ Ãâââ¬Â ÃÆâÃâÃâ Ãâââ¬Â C=n fold) was used [20]. The effects of stimulators were checked by screening at different time points where the effect on expression had the greatest impact.
Enzyme linked immunosorbent assay (ELISA)
Cell supernatant was centrifuged at 2000 RPM for 10 min at 4°C. The supernatant was stored at -80°C immediately. IL-8 level was measured by ELISA (Ebiosciences, California, USA). Minimum detectable level of ELISA kit was 4 pg/ml. An additional standard (IL-8 recombinant protein) was used to account for differences between ELISA assay performances. The assays were performed according to the manufacturer's instructions. The optical density was read using microplate reader at l=450 nm. Results were represented as pg/ml as mean of 3 samples (doublet) in each category.
Animals
Swiss albino male mice, 6-8 weeks old weighing 30-35 g were used. Mice were kept on 12 h/12 h light/dark cycle. The mice were fed standard chow formula and RO water ad labitum and allowed to acclimatize for one week. Each experimental group consisted of 5 mice. All the protocols were approved by the Institutional Animal Ethics Committee of the Jawaharlal Nehru University, New Delhi, India.
Induction of experimental colitis and bromelain treatment
Experimental colitis was induced in mice by administering 2% DSS (w/v) solution in RO water for 7 days following the protocol of Okayasu et al. [21]. Replacement with fresh DSS was done every third day. Pure bromelain (B5144, Sigma Aldrich, St. Louis, USA) at two different concentrations of 100 mg/kg/day and 200 mg/kg/day was administered orally during colitis induction. Mice were randomly divided into four groups, each with 5 mice: (1) non-treated control group, (2) group treated with only DSS (3) group co-treated with DSS and 100 mg/kg/day bromelain (4) group co-treated with DSS and 200 mg/kg/day bromelain.
Disease activity index (DAI)
During the period of 7 days, daily clinical evaluations included: weight loss, Hemoccult test or rectal bleeding and stool consistency. DAI was calculated by scoring changes described by Cooper et al. [22] and shown in Table 1.
| Scores | Percentage of weight loss (%) | Stool consistency | Hemoccult |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 1-5 | Loose stools | Hemoccult± |
| 2 | 6-10 | Loose stools | Hemoccult+ |
| 3 | 11-15 | Diarrhea | Hemoccult++ |
| 4 | 16-20 | Diarrhea | Rectal bleeding |
Table 1: Scoring parameters of Disease activity Index.
DAI score was graded on a scale of 0 to 4 and calculated as average of scores for weight loss, stool consistency, and rectal bleeding. All the above observations were made in a blinded fashion.
Colon length
Mice were sacrificed by cervical dislocation after each experiment and laparotomy was performed. Colon was excised, freed of adherent adipose tissue. Subsequent to washing in ice-cold 0.9% saline solution, the colon was placed on filter papers to measure their length. Colon length was measured from caecum to anus.
Myeloperoxidase assay (MPO assay)
Approximately 100 mg tissues from colon region of DSS treated mice were snap frozen in liquid nitrogen and homogenized in 1 ml of hexadecyltrimethyl ammonium bromide (HTAB) buffer dissolved in potassium phosphate buffer. Tissue particulate was discarded by centrifugation (5000 rpm, 2 min) and supernatant was collected. 10 µl of supernatant was taken in triplicate in 96 well plates. 10 µl HTAB buffer in triplicate was treated as blank. 200 µl of Potassium phosphate buffer (pH 6.0) containing 0.5 mM o-dianisidine dihydrochloride (MP Biochemicals Inc., Osaka, Japan) and 0.05% hydrogen peroxide was added. Optical densities were measured immediately at 450 nm at room temperature (25°C). Another reading was taken between 30 and 60 sec. Average of two readings (ÃÆâÃâÃâ Ãâââ¬Â A0-30 and ÃÆâÃâÃâ Ãâââ¬Â A30-60) was calculated and MPO was calculated using formula:
MPO (U/gm of tissue)=Average of ÃÆâÃâÃâ Ãâââ¬Â A0-30 and ÃÆâÃâÃâ Ãâââ¬Â A30-60/(time) × (MPO constant) × (tissue weight in gm)
MPO constant is 1.13 × 10-2
Hematoxylin and Eosin staining (H and E)
Thin (5 µm) sections of tissues embedded in wax were cut using microtome. Sections were kept on albumin or Poly-l-lysine coated slides and incubated at 37°C for 1 h. They were deparaffinized in 3 changes of xylene for 5 min each. Hydration of sections was done by giving changes of absolute, 90%, 70%, 50%, 30% and 10% alcohol for 1 min each. Sections were washed in tap water and stained in Harris's hematoxylin for 3 min. 1% acid alcohol was used for differentiation of sections. Changes in 10%, 30%, 50%, 70%, 90% and absolute alcohol for 1 min each was given for dehydration of sections. Sections washed with water were stained with eosin for 1 min. Sections were again dehydrated by alcohol change and mounted in D. P. X.
Histopathology
For histopathology analysis, a representative sample from the mid-part of the colon was fixed in 4% paraformaldehyde, embedded in paraffin, sectioned (5 µm), stained with hematoxylin and eosin (H and E), and examined at 20x magnification. The most affected part was scored by a person unaware of the treatments given. Inflammation was graded from 0 to 4 as per Gonzalez et al. [23] and shown in Table 2.
| Score | Description |
| 0 | No sign of inflammation |
| 1 | Low leukocyte infiltration |
| 2 | Moderate leukocyte infiltrate, thickening of the colon wall, moderate goblet cell loss, focal loss of crypts |
| 3 | Transmural infiltration, massive loss of goblet cells, diffuse loss of crypts |
Table 2: Grading of the histological scores given to Hematoxylin and Eosin stained colon section.
Statistical analysis
All data were analyzed using the paired student’s t-test. These data are presented as mean ± SEM, Data were analyzed by one way ANOVA test. A level of p<0.05 was considered significant. These exercises were done with GraphPad calculator available on www.graphpad.com/quickcalcs by GraphPad software Inc.
Results
In vitro anti-inflammatory action of bromelain in LPS-activated HT29 cells
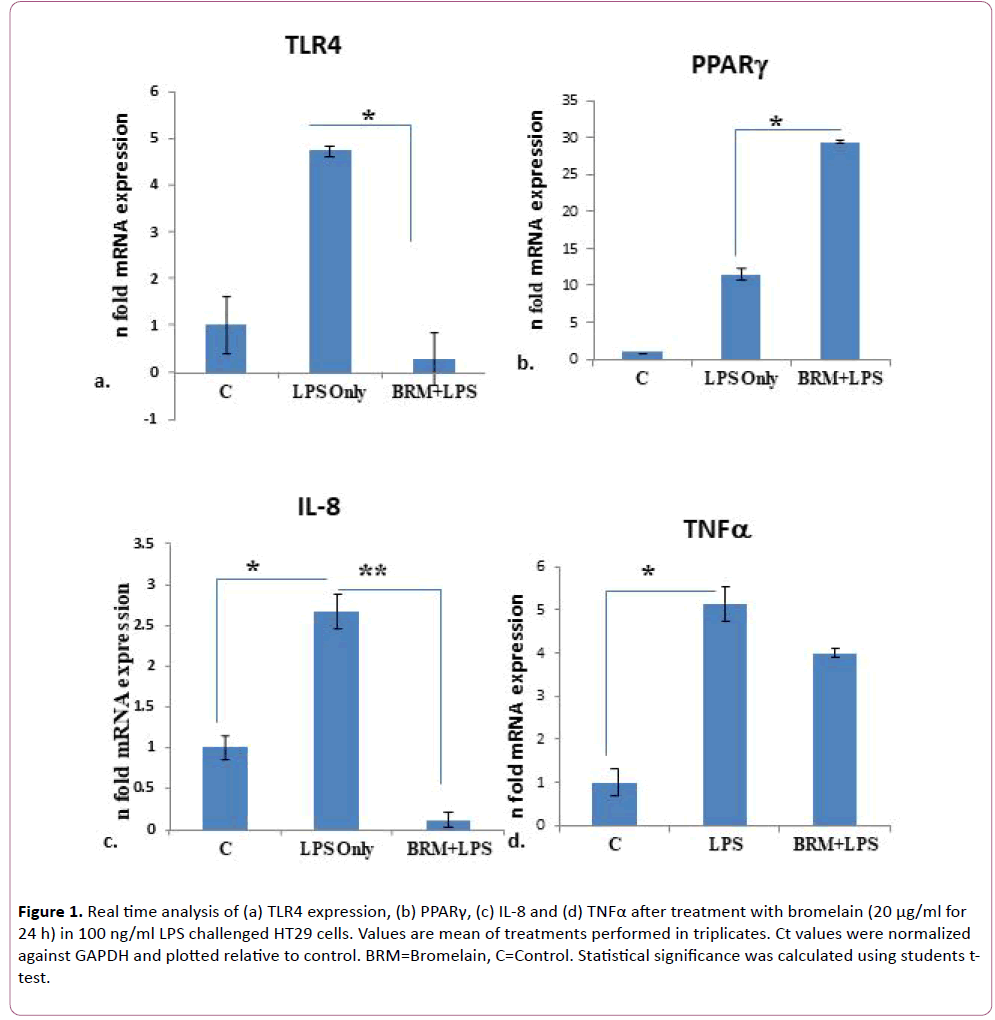
To check the anti-inflammatory effect of bromelain in vitro, HT29 cells were used since these intestinal cell lines are known to express TLR and PPARg receptors that are known to be important players in regulation of innate and adaptive immune responses in human colonic epithelium [24]. TLR4 expression was induced in HT29 cells by administering LPS treatment (100 ng/ml) for 24 h in 6-well plates followed by treatment with pure bromelain (20 µg/ml) for 24 h. Bromelain concentration was chosen based on a previous literature where 29 μg/ml of bromelain was found to inhibit the growth of HT29 cells [25]. This concentration of 20 μg/ml, therefore, ensured cell viability of HT29 cells. Total RNA was isolated and RT-PCR was performed for measuring the mRNA expression of TLR4, PPARg, and cytokines. Cell supernatant was also collected and stored -80°C for ELISA. As expected, the expression of TLR4 increased in LPS treated only as compared to untreated cells (Figure 1a). A 4.7 fold increase in mRNA expression was noted in HT29 cells treated only with LPS. However, when cells were post-treated with bromelain, mRNA expression of TLR4 decreased up to 0.28 fold as compared to the controls (Figure 1a). This change in TLR4 expression after bromelain treatment was statistically significant (p=0.04) as compared to the only LPS treated HT29 cells. It is well known that TLR4 activation of NFκB pathway negatively regulates PPARg expression. So we were curious to know whether bromelain treatment also simultaneously effects PPARg expression. Indeed fold change in PPARg mRNA expression was significantly higher (p=0.02) in LPS challenged HT29 cells post-treated with bromelain (29.4 fold) as compared to LPS challenged HT29 cells not treated with bromelain (11.5 fold) with respect to controls (Figure 1b).
Figure 1: Real time analysis of (a) TLR4 expression, (b) PPARγ, (c) IL-8 and (d) TNFα after treatment with bromelain (20 μg/ml for 24 h) in 100 ng/ml LPS challenged HT29 cells. Values are mean of treatments performed in triplicates. Ct values were normalized against GAPDH and plotted relative to control. BRM=Bromelain, C=Control. Statistical significance was calculated using students ttest.
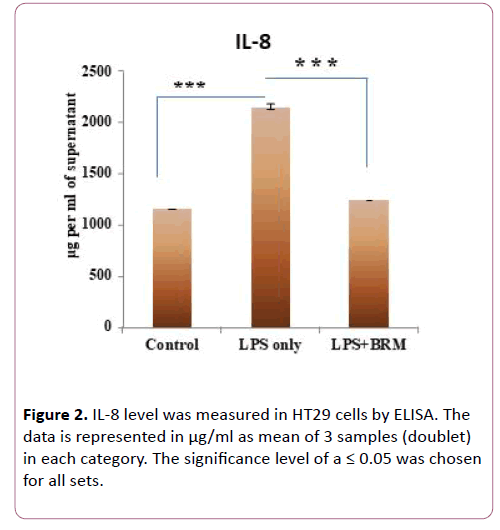
Since IL-8 production in the colonic epithelium induces neutrophil infiltration causing mucosal injury and is induced by TLR4 signaling [26], we measured the mRNA expression of cytokine IL-8. In cells treated with only LPS, IL8 mRNA expression increased by 2.7 fold as compared to untreated cells. Whereas in cells treated with bromelain post LPS challenge, IL8 expression was reduced to 0.11 fold (p=0.002) (Figure 1c). Similarly, TNFα, another TLR4 induced cytokine [27] also showed a tendency for decreased expression after bromelain treatment. As compared to untreated controls, TNFα mRNA expression increased by 5.1 fold in LPS treated cells whereas, after bromelain treatment to LPS induced cells, its expression increased by 4 fold only (p>0.05) (Figure 1d). We also validated expression of IL8 at the protein level by ELISA from cell culture supernatant as it showed significant mRNA level changes after LPS and bromelain treatments. We observed that IL-8 level was significantly elevated (2155 μg/ml of cell culture supernatant) when treated with LPS only (p=0.0002) as compared to untreated cells (Figure 2). Whereas after bromelain treatment to LPS induced cells, IL-8 protein levels reduced to 1250 μg/ml of cell culture supernatant (p=0.0002) when compared to LPS treatment only (Figure 2).
Bromelain ameliorates inflammation in vivo mice model
Effect of bromelain administered to DSS treated mice was studied using different parameters like disease activity index (DAI), colon length, inflammatory marker like myeloperoxidase (MPO) activity and histology.
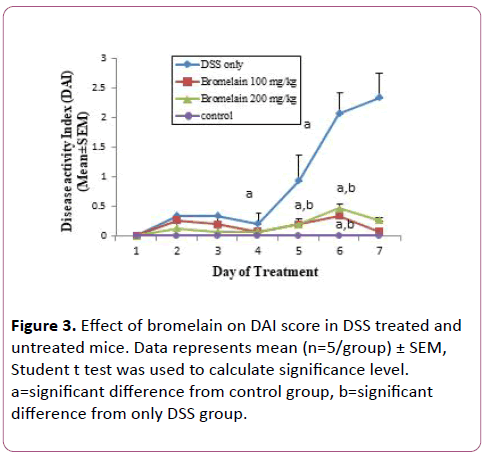
DAI scores decreased in DSS induced mice co-treated with bromelain
Administration of 2% DSS was found to cause significant clinical changes, which included weight loss, diarrhea, and the appearance of occult fecal blood. Bromelain drug was administered orally. Bromelain treatment improved parameters like diarrhea and intestinal bleeding in mice with DSS induced colitis. Consequently, significant reduction in DAI scores was observed in mice treated with bromelain (100 and 200 mg/kg/day for 7 days) (Figure 3). It was observed that bromelain in 100 mg dose was more effective in decreasing DAI scores as compared to 200 mg dose.
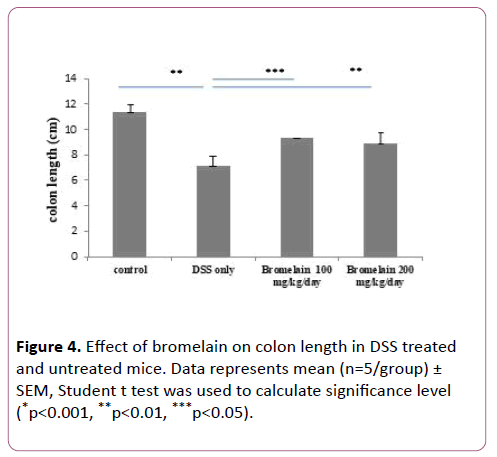
Bromelain prevented shortening of colon in colitis mice
Colon lengths were measured and compared to untreated, mice with DSS-induced colitis, and in mice co-treated with bromelain (100 and 200 mg/kg/day) and DSS. Significant shortening of colon length was observed in mice with DSS-induced colitis as compared to untreated. Oral administration of bromelain reduced shortening of colon length at both the concentrations 100 mg/kg/day and 200 mg/kg/day (Figure 4). It was observed that 100 mg dose was more effective in preventing colon shortening as compared to 200 mg dose. This effect was in correlation with DAI scores.
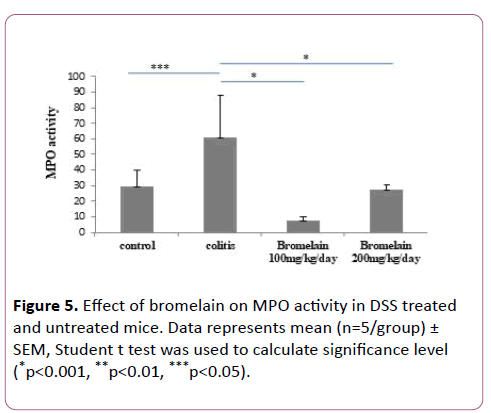
MPO levels were reduced in colitis mice treated with bromelain
Among markers of inflammation, an elevated level of myeloperoxidase was observed that correlated with the development of colonic inflammation during colitis. On administration of bromelain (100 and 200 mg/kg/day), MPO accumulation was significantly reduced in the colonic tissues of mice with DSS induced colitis (Figure 5). There was significant increase in MPO activity in only DSS treated mice as compared to control (untreated mice). Further, when compared with only DSS treated mice, bromelain-treated mice at a dose of 100 mg/kg/day (p<0.05) and at a dose of 200 mg/kg/day (p<0.001) showed significant decrease in MPO activity. 100 mg/kg/day dose was found more effective in preventing increase in MPO level compared to 200 mg (Figure 5).
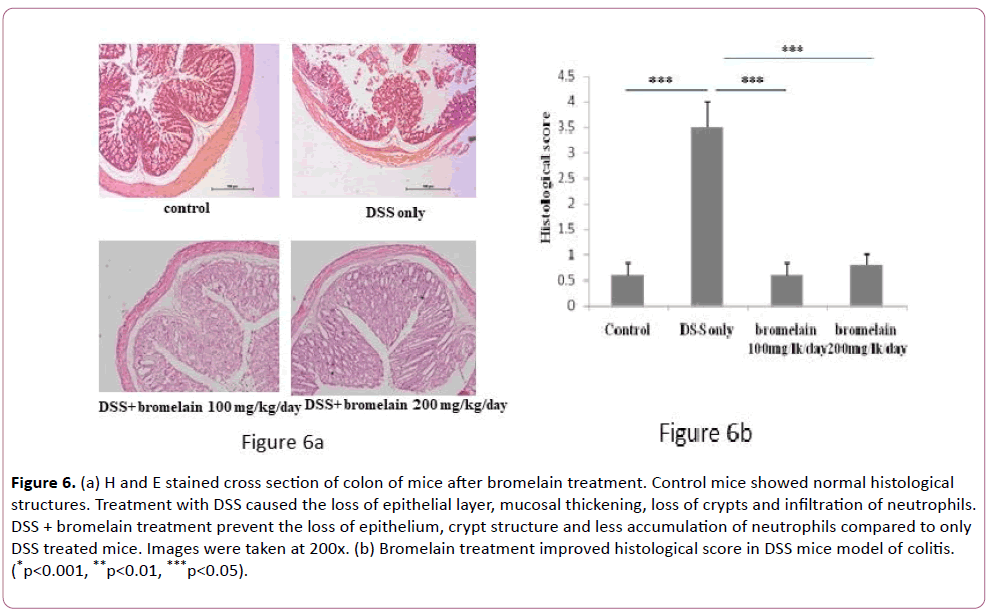
Pathological examinations of dissected colons were carried out by hematoxylin and eosin (H&E) staining and representative results are shown in Figure 6. Tissue sections from colon region exhibited distortion of epithelium in the crypt region, infiltration of inflammatory cells as expected in colitis-induced mice compared to control mice (Figure 6a). However, when the mice were co-treated with bromelain, the level of histopathological scores reduced significantly (Figure 6b). Interestingly, crypt structures were rather well-preserved and inflammatory reactions were significantly lower in tissue samples from mice treated in combination with DSS and bromelain (100 and 200 mg/kg/day) than only DSS treated mice (Figure 6a).
Figure 6: (a) H and E stained cross section of colon of mice after bromelain treatment. Control mice showed normal histological structures. Treatment with DSS caused the loss of epithelial layer, mucosal thickening, loss of crypts and infiltration of neutrophils. DSS + bromelain treatment prevent the loss of epithelium, crypt structure and less accumulation of neutrophils compared to only DSS treated mice. Images were taken at 200x. (b) Bromelain treatment improved histological score in DSS mice model of colitis. (*p<0.001, **p<0.01, ***p<0.05).
Discussion
Bromelain, a mixture of proteases derived from pineapple stem has been orally administered to UC patients and anecdotally observed to decrease inflammation during UC and induce remission in patients who are refractory to conventional therapy. According to a previous study, bromelain treatment in vitro decreased secretion of various pro-inflammatory cytokines from colon biopsies [12]. In order to investigate the effect of bromelain on expression of TLR4, PPARg and pro-inflammatory cytokines in vitro, we performed studies using HT29 cell line, an intestinal adenocarcinoma cell line. TLR 4 is a susceptibility gene of IBD as well as it is also over expressed during inflammation in IBD and signaling via TLR4 leads to secretion of pro-inflammatory cytokines [13,28]. PPARg is a nuclear receptor that is highly expressed in colon. It is an essential nuclear receptor that regulates cell proliferation, lipid metabolism, insulin sensitization and inflammation [29]. In contrast to TLR4 expression, PPARg expression is reported to decrease at mRNA and protein level in colon of UC patients [15,30]. PPARg represses NFkB mediated signaling in unstimulated cells, but after stimulation, TLR4 signaling through NFkB pathway down regulates PPARg expression [31]. Reciprocal relation between TLR4 signaling and PPARg expression has also been observed in IBD patients [32].
In our study, in LPS challenged HT29 cells TLR4 expression was upregulated as expected [33]. However, post-treatment with bromelain (20 µg/ml) resulted in significant down-regulation of TLR4 expression compared to LPS challenged cells not treated with bromelain. High expression of TLR4 in LPS challenged cells not treated with bromelain was consequently accompanied by low expression of PPARγ as compared to untreated HT29 cells. LPS challenged cells that were post-treated with bromelain resulted in significant up-regulation of PPARγ. This indicates that bromelain indirectly suppresses activation of the NFkB pathway by down regulating LPS induced TLR4 expression which in turn may lead to up regulated expression of PPARγ. As PPARγ suppresses NFkB activation the expression of pro-inflammatory cytokines like IL8 and TNFα also decreased after bromelain treatment in our study. These results are in accordance with a previous study where bromelain treatment decreased the secretion of various chemokines and cytokines including TNFα [12]. Bromelain has been reported to inhibit LPS induced NFkB activation in other cell lines as well [34].
In vivo studies in DSS induced colitis model represented high level of disease activity index (DAI) as expected [35]. Various studies which have used DSS model for validation of different therapeutic agents have reported that DSS-induced colitis is a reliable model for translation of data obtained in colitis model of mice to Ulcerative colitis occurring in humans [36]. Clinical manifestation of DSS colitis in acute phase included weight loss, diarrhea, occult blood in stools, However, treatment with bromelain decreased the scores of all the parameters of DAI score like rectal bleeding, improve in colon length and stool consistency. Some studies suggest that higher dosage of bromelain may be associated with lower tolerability, therefore, in our study, a dose of 100 mg of bromelain was found to be more effective in ameliorating the disease condition than 200 mg dose [37]. MPO level also decreased in mice effectively when treated with bromelain at a dose of 100 mg. MPO is present abundantly in monocytes and neutrophils. Increase in MPO activity is directly associated with increase in a number of these inflammatory cells at a particular site. We suggest bromelain may therefore also prevent accumulation of monocytes and neutrophils at the site of inflammation. A previous study by Fitzhugh et al. has reported that bromelain treatment decreases neutrophil migration during inflammation [38].
Typical histological changes of acute DSS-colitis observed by H and E staining were mucin depletion, epithelial degeneration, and necrosis leading to disappearance of epithelial cells. This was accompanied by neutrophils infiltration of lamina propria and submucosa as observed earlier by Melgar et al. [39]. Chronic colitis induced after 7 days of DSS has been reported to serve as a useful model to study the effects of pharmacologic agents in human inflammatory disease and mechanisms of perpetuation of inflammation [22]. Upon co-treatment with bromelain and DSS, we observed substantial improvement in the epithelial degeneration and necrosis and the mucin layer was also significantly less damaged. These findings complement previous findings where bromelain has been reported to have therapeutic benefits in many inflammatory diseases including IBD [12] and mice model of colitis [11].
Overall, our results show that bromelain modulates the expression of TLR4 and consequently of PPARγ in colon epithelial cells. Bromelain suppresses the activation of TLR4 signaling induced NFκB activation leading to diminished expression of downstream pro-inflammatory cytokines like IL8 and TNFα. Bromelain moreover ameliorates DSS induced histological inflammation and clinical features like diarrhea in colitis model of mice apart from reducing MPO activity. These data collectively indicate that bromelain has potential to be used either as a supplement or as an alternative therapy in UC patients.
References
- Sartor RB (1997) Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol 92: 5S-11S.
- Verma N, Ly H, Liu M, Chen J (2016) Intraneuronal amylin deposition, peroxidative membrane injury and increased IL-1β synthesis in brains of Alzheimer's disease patients with Type-2 Diabetes and in diabetic HIP Rats. J Alzheimers Dis 53: 259-272.
- Liu M, Verma N, Peng X, Srodulski S, Morris A, et al. (2016) Hyperamylinemia increases IL-1β synthesis in the heart via peroxidative sarcolemmal injury. Diabetes 65: 2772-2783.
- Verma N, Verma R, Kumari R, Ranjha R, Paul J, et al. (2014) Effect of salicin on gut inflammation and on selected groups of gut microbiota in dextran sodium sulfate induced mouse model of colitis. Inflamm Res 63: 161-169.
- Neurath M, Fuss I, Strober W (2000) TNBS-colitis. Int Rev Immunol 19: 51-62.
- Wirtz S, Neurath MF (2007) Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 59: 1073-1083.
- Wirtz S, Neufert C, Weigmann B, Neurath MF (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546.
- Rowan AD, Buttle DJ, Barrett AJ (1990) The cysteine proteinases of the pineapple plant. Biochem J 266: 869-875.
- Izaka K I, Yamada M, Kawano T, Suyama T (1972) Gastrointestinal absorption and anti-inflammatory effect of bromelain. Jpn J Pharmacol 22: 519–534.
- Taussig S, Batkin S (1988) Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J Ethnopharmacol 22: 191-203.
- Hale LP, Greer PK, Trinh CT, Gottfried MR (2005) Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin Immunol 116: 135–142.
- Onken JE, Greer PK, Calingaert B, Hale LP (2008) Bromelain treatment decreases secretion of pro-inflammatory cytokines and chemokines by colon biopsies in vitro. Clin Immunol 126: 345–352.
- Cario E (2010) Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis 16: 1583-1597.
- Sipos F, FÃÆââ¬Â¦Ãâñr I, Constantinovits M, Tulassay Z, MÃÆââ¬Â¦Ãâñzes G (2014) Contribution of TLR signaling to the pathogenesis of colitis-associated cancer in inflammatory bowel disease. World J Gastroenterol 20: 12713–12721.
- Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, et al. (2003) Impaired expression of peroxisome proliferator-activated receptor γin ulcerative colitis. Gastroenterology 124: 1265–1276.
- Shen W, Gao Y, Lu B, Zhang Q, Hu Y, et al. (2014) Negatively regulating TLR4/NF-κB signaling via PPARα in endotoxin-induced uveitis. Biochim Biophys Acta 1842: 1109-1120.
- Chen Y, Hu M, Lin AJ, Jenkins AC, Keech AC, et al. (2013) Therapeutic effects of PPAR alpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62: 261–272.
- Tyagi S, Gupta P, Saini A, Kaushal C, Sharma S (2011) The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res 2: 236-240.
- Vidal K, Donnet-Hughes A, Granato D (2002) Lipoteichoic acids from Lactobacillus johnsonii strain La1 and Lactobacillus acidophilus strain La10 antagonize the responsiveness of human intestinal epithelial HT29 cells to lipopolysaccharide and gram-negative bacteria. Infect Immun 70: 2057-2064.
- Kenneth JL, Thomas DS (2001) Analysis of relative gene expression data using real-time PCR and the 2-ÃÆâÃâÃâ Ãâââ¬Â ÃÆâÃâÃâ Ãâââ¬Â C method. Method 25: 402-408.
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, et al. (1990) A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98: 694-702.
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238-249.
- Gonzalez-Rey E, Chorny A, Delgado M (2006) Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 130: 1707–1720.
- Pedersen G (2015) Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium. Dan Med J 62: B4973.
- Amini A, Ehteda A, Masoumi Moghaddam S, Akhter J, Pillai K, et al. (2013) Cytotoxic effects of bromelain in human gastrointestinal carcinoma cell lines (MKN45, KATO-III, HT29-5F12, and HT29-5M21). Onco Targets Ther 6: 403-409.
- Eskan MA, Rose BG, Benakanakere MR, Zeng Q, Fujioka D, et al. (2008) TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells. Eur J Immunol 38: 1138-1147.
- Hoareau L, Bencharif K, Rondeau P, Murumalla R, Ravanan P, et al. (2010) Signaling pathways involved in LPS induced TNFalpha production in human adipocytes. J Inflamm (Lond) 7: 1.
- Meena NK, Verma R, Verma N, Ahuja V, Paul J (2013) TLR4 D299G polymorphism modulates cytokine expression in ulcerative colitis. J Clin Gastroenterol 47: 773-80.
- Laudet V, Auwerx J, Gustafsson JA, Wahli W (1999) Letter to the Editor A Unified Nomenclature System for the Nuclear Receptor Superfamily. Cell 97: 161-163.
- Yamamoto-Furusho JK, Peñaloza-Coronel A, Sánchez-Muñoz, F, Barreto-Zuñiga, Dominguez-Lopez A (2011) Peroxisome proliferator-activated receptor-gamma (PPAR-γ) expression is downregulated in patients with active ulcerative colitis. Inflamm Bowel Dis 17: 680-681.
- Necela BM, Su W, Thompson EA (2008) Toll-like receptor 4 mediates cross-talk between peroxisome proliferator-activated receptorÃÆâÃâââ¬Ãâïg and nuclear factor-kB in macrophages. Immunology 125: 344–358.
- Annese V, Rogai F, Settesoldi A, Bagnoli S (2012) PPARg in inflammatory bowel disease. PPAR Research.
- Vaure C, Liu YA (2014) Comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol 5: 316.
- Hou RCW, Chen YS, Huang JR, Jeng KCG (2006) Cross-linked bromelain inhibits lipopolysaccharide-induced cytokine production involving cellular signaling suppression in rats. J Agric Food Chem 54: 2193–2198.
- Wirtz S, Neufert C, Weigmann B, Neurath MF (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546.
- Melgar S, Karlsson L, Rehnström E, Karlsson A, Utkovic H (2008) Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol 8: 836-844.
- Brien S, Lewith G, Walker A, Hicks SM, Middleton D (2004) Bromelain as a Treatment for Osteoarthritis: a Review of Clinical Studies. Evid Based Complement Alternat Med 1: 251-257.
- Fitzhugh DJ, Shan S, Dewhirst MW, Hale LP (2008) Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin Immunol 128: 66–74.
- Melgar S, Karlsson A, Michaelsson E (2005) Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol 288: G1328-G1338.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences