The Role of T-Regulatory Expression in Autoimmune Thyroid Disease and its Association with Thyroid Antibody
Dwitya Elvira
Dwitya Elvira*
Internist Medical Faculty of Andalas University, Padang, West Sumatra, Indonesia
- *Corresponding Author:
- Dwitya Elvira
Internist Medical Faculty of Andalas University
Padang, West Sumatra
Indonesia
Tel: +62-751-71181
Email: dwitya.elvira.de@gmail.com
Received date: April 15, 2016; Accepted date: May 07, 2016; Published date: May 13, 2016
Citation: Elvira D. The Role of T-Regulatory Expression in Autoimmune Thyroid Disease and its Association with Thyroid Antibody. J Autoimmun Disod. 2016, 2:2. DOI:10.21767/2471-8513.100019
Copyright: © 2016 Elvira D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Autoimmune thyroid disease is one of the most common organ-specific autoimmune disorders, in which Graves' disease (GD) and Hashimoto Thyroiditis (HT) were the most common clinical expressions. GD is characterized by hyperthyroidism due to excessive production of thyroid hormone induced by TSH receptor antibody. Imbalance between Th1/Th2 cells in producing antibodies that regulated by T-regulator cell believed to be the cause of this disease. The aim of this study was to evaluate prospectively the relationship between Tregulator cell and thyroid antibody (TRAb) of thyroid autoimmune disorder, especially Graves' disease. Method: T-regulator (T-reg), thyroid antibody (TRAb), thyroid hormone; FT4 (free thyroxine) and TSH of peripheral blood samples from Graves' disease patient was analyzed using ELISA. T-regulator cells were identified using Human Forkhead Box P3 (Foxp3) kit provided by Cloud-Clone Corp. Furthermore, statistical analysis was conducted using SPSS 21 software. Result: There was significant correlation between T regulator cell expression and thyroid antibody (TRAb) in Graves' disease patient (p<0.05). In our study, T-regulator cell expression using Human FoxP3 kit was found increase with mean serum 23.51 pg/mL (p value >0.05; reference range 0.312-20 pg/mL) while TRAb was also found increased with mean serum 5.63 pg/mL (p value >0.05, reference range <0.1 pg/mL). Conclusion: Expression of T-regulator cells and increased of TRAb levels may be involved in the pathogenesis of disease through cytokine produced by T-reg cells, such as TGF-β and IL-10, not only by the quantity of T-reg expression.
Keywords
Autoimmune thyroid; Graves' disease; Tregulator cells; TRAb
Introduction
Regulatory T cells (Treg) are a subset of CD4+ T cells that express CD25 function as a regulator balance of Th1/Th2 with involvement inhibitor molecules such as cytokines; TGF-β, IL-10 and its carrier (Lrrc 32, aka GARP), expression of IL-2 (IL2Rα, CAG-3, CTLA4) as well as a number of other inhibitor molecules. In autoimmune conditions, the levels of Treg can be differ on variety of illnesses, which can be found an increase, decline on number or no different from healthy controls [1-3].
Thyroid is a hormone-producing gland most often had an autoimmune condition than any organ. Clinically, the most common presentations of autoimmune thyroid disease (AITD) are Graves’ hyperthyroidism and hypothyroidism caused by Hashimoto’s disease. Both diseases are characterized by lymphocytic infiltration of the thyroid and the production of thyroid autoantibodies. In Graves’ disease, thyrotoxicosis results from the production of stimulating antibodies to the TSH receptor, whereas Hashimoto’s disease is characterized by tissue destruction and consequent hypothyroidism [1-4].
Graves’ Disease (GD) is a thyroid autoimmune disease which is the most prevalent cause of hyperthyroidism in adults characterized by hyperthyroidism, diffuse goiter and presence of thyroid antibody (TSHR Ab). Incidence of Graves’ disease reached 4% of the population, while in Indonesia, based on RISKESDAS 2013, showed an increased incidence to 0.4% of population. Although only small percentage, but in quantities it’s large enough and tend to increase annually [4-6].
Thyrotoxicosis, which occurs in GD patients, may cause systemic clinical manifestations that may increase morbidity and mortality. Overproduction of B cells in GD caused by imbalance of Th1/Th2 that normally regulated by Treg. Stimulation of the thyroid stimulating hormone receptor (TSHR) that causes a hyper function of the thyroid gland that manifesting in GD as thyrotoxicosis. Presence of TSHR antibody (TRAb) is a disease marker in GD patients, which also present as dominant autoantibody in Graves’ disease [5-8].
In this study, we aimed to identifying the levels of regulatory T cells (Treg) and to investigate its correlation to the levels of TSH receptor antibody (TRAb) in GD patients.
Material and methods
Patients
A total of 30 patients newly diagnosed as Graves’ disease based on clinical and laboratory examinations that went to Endocrinology and Diabetes Mellitus Outpatient of M. Djamil Hospital, Padang, Indonesia were included in this study. Exclusion criteria of this study were patients who had received treatment of GD as well as history of other autoimmune or infections. Age, gender, and goiter size (volume and gradation) were subsequently recorded as baseline characteristic of study. Goiter volume assessed using ultrasonography thyroid and goiter gradation classified into three grades (grade I: palpable; grade II: palpable and visible; grade III: visible from distance).
Laboratory measurement
All subjects had blood sample collected without anticoagulants and the serum was stored at minus 20°C until needed. Free thyroxin (FT4), TSH and TRAb were measured using ELISA method test. T-regulatory cells were measured using FOXP3, a transcription factor of Treg cells which also took a special role as biomarker of Treg cells, also with ELISA method test. The normal range of FT4 and TSH in our laboratory was 9-20 nmol/L and 0.25-5 IU/ml, respectively. Serum TRAb were determined using Human TRAb ELISA Kit from EIAab Wuhan Science Co. We considered positive all values greater than 1 pg/mL. Serum Treg FOXP3 were determined using FOXP3 (forkhead box protein 3) Human ELISA Kit by Cloud-Clone Corp and normal value was 0.3 -20 pg/mL.
Statistical analysis
Data were analyzed using SPSS Program version 21 (SPSS Inc.). Correlation between T-regulatory and TRAb were analyzed by Pearson correlation test, if data normally distributed and Spearman-rank test if the data didn’t normally distribute.
Results
The aim of this study was to investigate correlation between T-regulatory (Treg) cell using FOXP3 marker with thyroid antibody in Graves’ disease, TRAb. As shown in , Graves’ disease was found the most in female gender than male (7:3) and age of GD patients were found between 19-74 years old, with mean of age were 40.7 ± 14.81 years old. Volume and gradation of goitre also shown in Table 1.
| Characteristic | n = 30 | (%) |
| Age | ||
| < 30 | 9 | 30 |
| 30-39 | 6 | 20 |
| > 40 | 15 | 50 |
| Gender | ||
| Male | 10 | 33.3 |
| Female | 20 | 66.7 |
| Volume (gr) | ||
| < 10 | 24 | 80 |
| Oct-15 | 5 | 16.7 |
| > 15 | 1 | 3.3 |
| Gradation | ||
| Grd. I | 7 | 23.3 |
| Grd. II | 12 | 40 |
| Grd. III | 11 | 36.7 |
Table 1: Baseline Characteristic of Graves’ disease patients.
T-regulatory and thyroid antibody, TRAb were found increased from normal value on this study, which the levels of Treg was 23.51 ± 15.7 pg/mL, while the levels of TRAb was 5.63 ± 3.72 pg/mL as shown in Table 2.
| Variabel | Mean ± SD | Normal Range |
| T-reg (pg/ml) | 23.51 ± 15.7 | 0.3-20 |
| TRAb (pg/ml) | 5.63 ± 3.72 | <1 |
| FT4 (nmol/L) | 74.28 ± 61.09 | 09-20 |
| TSH (IU/ml) | 0.1 ± 0.65 | 0.25-5 |
Table 2: Results of T-regulatory cell, TRAb and thyroid hormone in Graves’ disease patients.
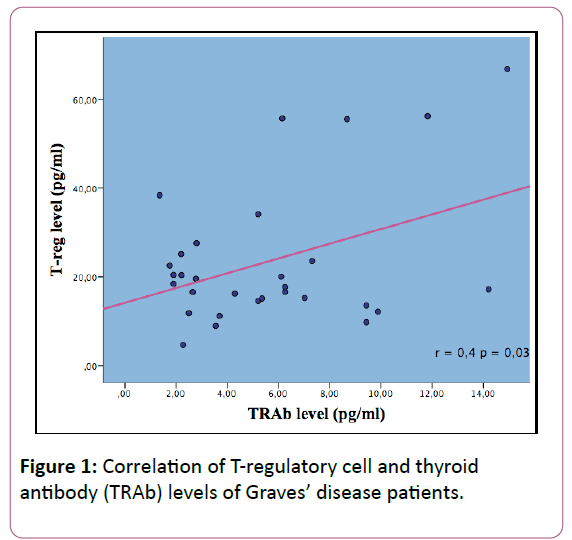
To investigate the correlation between T regulatory and TRAb, we were using Spearman rank test because the data didn’t distribute normally. There was significant positive correlation between Treg and TRAb levels, with r=0.04, p < 0.05. The correlation graphic as seen in Figure 1.
Discussion
Baseline characteristic
Our research shown that Graves’ disease affects more in woman than man, which is about 77%. Similar result was obtained by Marina, Hartono, Mao and Zhu that shown GD affect woman than man. Based on literature, autoimmune disease occurs more in woman than in man and this may be attributable to the role of estrogens, whereas estrogen promote autoimmune diseases with a type 2 cytokine profile, although how sex hormone plays a role in modulating cellular and molecular remain elusive [7-11].
Onset age of GD patients in our study was varied from age 19 to 74 years old, mostly found in the age over 40 years old. Marina studies had shown the same result with our study that 50% patients were over 40 years old. Mao shown that mean of age of 77 patient of GD were 41.1 ± 12.7 years old [7,9].
Correlation between T-regulatory cells ant thyroid antibody of Graves’ disease
The current studies have revealed a novel and potentially marker of FOXP3 serum analysis to investigate the correlation between T-regulatory cells (Treg) and TSHR autoantibodies (TRAb) in Graves’ disease patients. T-regulatory cells act as a counterbalance of T effector cell activity for immune homeostasis. Function or dysfunction of Tregs plays pivotal role in autoimmune disease, with one of the important function is the immunosuppressive regulation of autoreactive T cell. More recently, the fork head transcription factors FOXP3 has been shown to specifically expressed in Tregs and to be a central control element in Treg development and function [12-14].
Classical biochemical features of hyperthyroid GD (elevated thyroid hormone levels and low to undetectable TSH) arise from the action of TSHR stimulating antibodies which can be detected in virtually all untreated patients using 3rd generation immunometric receptor assay.
On this study, we found that Treg cells were slightly increased in GD patients from normal range (0.3-20 IU). Previous studies shown that proportion of Treg cells in peripheral blood reported to be increase, decrease or remain unchanged in several autoimmune diseases. Our study shows consistent result with the conclusion from Pan et al. and Wang et al., but in contrast to the reports from Nakano et al., Valencia et al., and Mao et al. Hongxiang et al. study shown unchanged of Treg cells in GD patients compared to healthy control (p>0.05). These finding suggest that Treg cells not only depend on the number of cells, but also depend on the function of Treg itself in secreting molecular inhibitor cytokine such as TGF-β, IL-10 and IL-35. Other than number and function of Treg cells, disruption of tolerance also present as a matter that affect T cell effector resistance to inhibition activity. Studies by Schneider et al. and Lawson et al. had shown Treg cell resistance in GD. The same condition also develops on other autoimmune disease such as SLE and rheumatoid arthritis [7,15-20].
On this study, we found increasing of TRAb serum in 30 GD patients range between 1.36–14.93 IU/l with mean 5.63 ± 5.21 IU/l. Increasing of TRAb level were found in Skowronck et al. and Klatka et al., Zhu et al. and Mao shown slightly increase of TRAb level (24.21 ± 6.06 IU/l), while Hu shown the highest (91.41 ± 22.62 IU/l). Difference between these studies might be caused by the difference of sample quantity and subject characteristic such as age, race and methods of study [7,11,21-23].
The main finding of this study is to investigate the correlation between Treg cells using FOXP3 marker and thyroid antibody of Graves’ disease. Correlation between Treg with TRAb in this study revealed significant correlation (p< 0.05; r=0.04). Positive correlation between these variable shown that the higher level of Treg, contribute to the overproduction of autoantibody (TRAb) level that will lead to clinical manifestations of Graves’ disease such as hyperthyroidism, diffuse goiter and exophtalmus. Studies that evaluate this relationship still rarely found. Mao studies shown the similar results as our study, whereas increasing of Treg also increase antibody levels in GD (p< 0.05). Bossowski et al. and Zhu et al. shown significant correlation with p=0.01 and r=0.04 [7,11,24].
The significant correlation of these variable showed that there is a pivotal role of regulatory T cell in autoimmune pathogenesis especially Graves’ disease. Since 1990, Treg cells had been known as CD4+ Th subsets which have an important role in suppressing both innate and adaptive immune responses. The naturally occurring Tregs have a major role in modulating the activity of self-reactive cells. Treg cells modulate potentially self reactive T cells through secreting suppressive cytokines or direct cell-contact-dependent mechanism. The dysfunction or depletion of this cell type contributes to autoimmune diseases like autoimmune thyroid. Some evidences suggest that patients with autoimmune diseases may have remarkably reduced number of Treg cells with CD4+CD25. Moreover, recently, it has been shown that the upregulation of Treg cells can suppress experimental autoimmune thyroiditis. As mentioned earliar, Treg levels vary in some autoimmune diseases. This difference is influenced by various factors such as different characteristic of basic research data (race, age and number of samples) and markers are used, while several other studies using the CD4+CD25+ Treg with flow cytometry method. Identification of Foxp3 as the critical determinant for Tregs development and function has generated expanded interest in studying balance between autoimmunity and regulatory mechanism in human autoimmune disease, especially GD. Studies also has been expanded to identification of Treg genotype, Foxp3 gene as a crucial factor in contribution of Graves’ disease pathogenesis [25].
According to the literature, in addition to the role of Treg, non-genetic factors and the environment also affects the occurrence of GD, such as smoking history, excessive iodine consumption, infection or stress. Number of studies shown that excessive consumption of iodine was able to stimulate overproduction of thyroid antibodies, as well as increase the risk of autoimmune thyroid disease. Data source RISKESDAS 2013 showed that as many as 30.4% of children aged 6-12 years 24.9% of women of childbearing age, 21.3% and 18.3% of pregnant women breastfeeding mothers have an increased risk of excessive iodine which increase the susceptibility of GD. Vitale et al. showed that the iodine content overload can induce apoptosis of cells of the thyroid gland through a mechanism involving the formation of free radicals, which, according to Bagchi et al. was the onset of autoimmune thyroid disease as the occurrence in Graves disease [26,27].
Conclusion
Based on this study, there is a significant association between high levels of Treg with thyroid autoantibodies, TRAb. This result showing that Tregs play a role in the pathogenesis of Graves' disease, in addition to genetic and other environmental factors. Further research is needed to look at the role of cytokines produced by Treg such as TGF, IL-10 and IL-35 in association with the pathogenesis of Graves' disease.
References
- Benoist C, Mathis D (2012) Treg cells, life history, and diversity. Cold Spring Harb Perspect Biol 4: a007021.
- Vignali DA, Collison LW, Workman CJ (2008) How regulatory T cells work. Nat Rev Immunol 8: 523-532.
- Shevach EM (2009) Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30: 636-645.
- De Groot LJ, Beck-Peccoz P, Chrousos G. Graves’ disease and manifestations of thyrotoxicosis.
- Jurecka-Lubieniecka B, Ploski R, Kula D, Krol A, Bednarczuk T, et al. (2013) Association between age at diagnosis of Graves' disease and variants in genes involved in immune response. PLoS One 8: e59349.
- Riset Kesehatan Dasar 2013 (2013) Badan Penelitian dan Pengembangan Kesehatan Kementerian Kesehatan RI.
- Mao C, Wang S, Xiao Y, Xu J, Jiang Q, et al. (2011) Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves' disease. J Immunol 186: 4734-4743.
- Stefana M, Weic C, Lombardia A, Lia CW, Concepciona ES, et al. (2014) Genetic–epigenetic dysregulation of thymic TSH receptor gene expression triggers thyroid autoimmunity. PNAS 111: 12562-12567.
- Marina (2011) Thesis: Peran Propiltiourasil sebagai terapi inisial terhadap kadar T3, T4, TSH dan IL-4 pada penyakit. Graves: 1-99.
- Hartono A (2010) Thesis: Hubungan kadar interleukin-4 dengan Triiodotironin (T3), Tiroksin (T4) dan Tiroid Stimulating Hormon (TSH) serum pada penyakit Graves:1-78.
- Zhu C, Ma J, Liu Y, Tong J, Tian J, et al. (2012) Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab 97: 943-950.
- Kang SW, Kim SH, Lee N, Lee WW, Hwang KA, et al. (2012) 1.25-Dihydroxivitamin D3 promotes FOXP3 expression via binding to vitamin D response element in its conserved noncoding sequence region. J Immunol 188: 5276-5282.
- Jiang W, Zheng L, Xu L, Zhang Y, Liu X, et al. (2015) Association between FOXP3, FOXE1 gene polymorphisms and risk of differentiated cancer in Chinese Han population. Mol Biol 4: 1-5.
- Inoue N, Watanabe M, Morita M, Tomizawa R, Akamizu T, et al. (2010) Association of functional polymorphisms related to the transcriptional level of FOXP3 with prognosis of autoimmune thyroid diseases. Clin Exp Immunol 162: 402-406.
- Pan D, Shin YH, Gopalakrishnan G, Hennessey J, De Groot LJ (2009) Regulatory T cells in Graves' disease. Clin Endocrinol (Oxf) 71: 587-593.
- Wang L, Liu Y, Han R, Beier UH, Bhatti TR, et al. (2015) FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3. J Clin Invest 125: 1111-1123.
- Nakano A, Watanabe M, Iida T, Kuroda S, Matsuzuka F, et al. (2007) Apoptosis-induced decrease of intrathyroidal CD4(+)CD25(+) regulatory T cells in autoimmune thyroid diseases. Thyroid 17: 25-31.
- Valencia X, Yarboro C, Illei G, Lipsky PE (2007) Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 178: 2579-2588.
- Wang H, Zhao S, Tang X, Li J, Zou P (2006) Changes of regulatory T cells in Graves' disease. J Huazhong Univ Sci Technolog Med Sci 26: 545-547.
- Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, et al. (2008) The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol 181: 7350-7355.
- Ben-Skowronek I, Szewczyk L, Kulik-Rechberger B, Korobowicz E (2013) The differences in T and B cell subsets in thyroid of children with Graves' disease and Hashimoto's thyroiditis. World J Pediatr 9: 245-250.
- Klatka M, Grywalska E, Partyka M, Charytanowicz M, Kiszczak-Bochynska E, et al. (2014) Th17 and Treg cells in adolescents with Graves' disease. Impact of treatment with methimazole on these cell subsets. Autoimmunity 47: 201-211.
- Hu Y, Tian W, Zhang LL, Liu H, Yin GP, et al. (2012) Function of regulatory T-cells improved by dexamethasone in Graves' disease. Eur J Endocrinol 166: 641-646.
- Bossowski A, Moniuszko M, DÄ…browska M, Sawicka B, Rusak M, et al. (2013) Lower proportions of CD4+CD25(high) and CD4+FoxP3, but not CD4+CD25+CD127(low) FoxP3+ T cell levels in children with autoimmune thyroid diseases. Autoimmunity 46: 222-230.
- Mai J, Wang H, Yang XF (2010) Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front Biosci (Landmark Ed) 15: 986-1006.
- Vitale M, Di Matolla T, D’ascoli F, Salzano S, Bogazzi F, et al. (2000) Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinology 141: 598-605.
- Bagchi N, Brown TR, Sundick RS (1995) Thyroid cell injury is an initial event in the induction of autoimmune thyroiditis by iodine in obese strain chickens. Endocrinology 136: 5054-5060.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences