The Spectrum of Underlying Diseases in Patients with Positive-Direct Antiglobulin Test
Riad Akoum1*, Michel Saade1, Wassim Serhal2 and Emile Brihi3
1Department of Medical Oncology, Lebanese American University Medical Center-Rizk Hospital, Beirut, Lebanon
2Department of Laboratory Medicine, Lebanese American University Medical Center-Rizk Hospital, Beirut, Lebanon
3Department of Radiation Oncology, Lebanese American University Medical Center-Rizk Hospital, Beirut, Lebanon
- *Corresponding Author:
- Riad Akoum
Department of Medical Oncology,
Lebanese American University,
Medical Center-Rizk Hospital, Beirut,
Lebanon,
Email: riad.akoum@laumcrh.com
Received date: March 15, 2023, Manuscript No. IPADO-23-16105; Editor assigned date: March 17, 2023, PreQC No. IPADO-23-16105 (PQ); Reviewed date: March 31, 2023, QC No. IPADO-23-16105; Revised date: April 07, 2023, Manuscript No. IPADO-23-16105 (R); Published date: April 14, 2023, DOI: 10.21767/2471-8513.09.01.30
Citation: Akoum R, Saade M, Serhal W, Brihi E (2023) The Spectrum Of Underlying Diseases in Patients With Positive-Direct Antiglobulin Test. J Autoimmune Disord Vol.9.No.1: 30.
Abstract
Background: The association between a positive Direct Antiglobulin Test (DAT) and malignancy has been recognized. However, there has been dissimilar data on the spectrum of underlying malignancies and the timing of occurrence.
Objective: To estimate the pattern of underlying diseases in adult patients with warm reactive autoantibodies over a 12 year-period.
Methods: All consecutive patients with positive-DAT were selected and a follow-up for concomitant or subsequent development of malignancy or infectious or autoimmune disease was conducted. The gel technique with 5 monospecific anti-human globulin agents: Anti-IgG, anti-IgA, anti-IgM, anti-C3c and anti-C3d were used.
Results: Between 2009 and 2019, 164 patients, 86 males and 77 females, were found to have a positive DAT due to warm autoantibody among 12200 patients subjected to testing. The mean age was 62 years. Seventy two patients had Autoimmune Hemolytic Anemia (AIHA). The spectrum of underlying disease included: Lymphoproliferative disorders (14%), myeloproliferative disorders (10.3%), solid tumors (18.3%), autoimmune diseases (11%) and infectious disease. The most frequently observed condition was myelodysplastic syndrome at all stages.
The strength of DAT reaction was correlated with the degree of hemolysis but not with the stage of malignancy. All patients with AIHA were given corticosteroids, 3 of them required second line rituximab.
Conclusion: A positive-DAT with or without AIHA suggest the presence of underlying malignancy, mainly hematological and even in early stages. All patients with MDS should be tested for DAT for diagnostic and therapeutic issues.
Keywords
Positive-DAT; Myelodysplastic syndrome; Underlying malignancy
Introduction
The Direct Antiglobulin Test (DAT) is used to detect the presence of red blood cell- coated antibody and/or complement. Routine DAT is performed using polyspecific antiglobulin reagent containing anti-IgG, IgA, IgM, C3d and C3b. Once a positive-DAT is found, monospecific reagents; anti-IgG, anti-C3b+C3d, anti- IgM, anti IgA are used to determine the specific type of protein bound to the RBC’s membrane.
In the presence of clinical and biological stigmata of hemolysis, DAT may help in diagnosing Autoimmune Hemolytic Anemia (AIHA). A positive-DAT without evidence of hemolysis has been reported in 0.1% to 1.4% of blood donors and 1% to 3.5% of unselected hospitalized patients [1-5]. AIHA may be primary or associated with an underlying disease. It is a rare event occurring in 1/35000 to 1/80000 individuals yearly at any age and has female predominance [6,7]. The idiopathic form occurs mostly between 15 and 40 years whereas the secondary form occurs later in life. According to the first largest published studies 41% to 65% of AIHA were considered to be idiopathic, 22% to 40% secondary to underlying diseases and 15% to 19% drug-induced [7,8].
The most common types of autoantibodies involved in AIHA are warm antibodies (75%-90%) that react optimally with human RBCs at 370°C. In warm AIHA, the DAT is positive with IgG in 20% to 66%, with IgG and complement component C3d in 24% to 64% and with C3d alone in 7% to 14% of cases [9-12]. There is a recognized bi-directional relationship between Autoimmune Disease (AID) and lymphoproliferative disorders; especially Non- Hodgkin’s Lymphoma (NHL) and Chronic Lymphocytic Leukemia (CLL) [13-17]. Myeloproliferative Neoplasms (MPN) and myelofibrosis have also long been associated with AIHA [18-20].
A positive DAT in patients with Multiple Myeloma (MM) was most frequently observed with IgG and usually produced little hemolysis [21].
The increased incidence of a positive DAT in Myelodysplastic Syndrome (MDS), up to 50%, is thought to be a manifestation of disturbed immune homeostasis and might support a more rational use of steroid therapy in these patients [22-30].
Positive-DAT was found in 11% of AML patients at the time of diagnosis regardless of FAB morphological subtype and may develop subsequently during remission or progression [31].
It is known that solid malignant tumors can trigger the development of auto-antibodies against proteins in different organ systems that cause paraneoplastic syndromes. In many AID autoantibodies may develop against multiple organs including the blood system. The availability of DAT has rendered the diagnosis of AIHA easier however the mechanism by which this self-tolerance is deregulated remains still not fully understood.
An underlying disease has been found in 51% to 77% of positive DAT-cases with or without AIHA [32-36]. Limited data has been reported on the timing between the diagnosis of positive-DAT and the occurrence of underlying malignancy. Genty, et al. have studied the bone marrow of patients with AIHA and were able to show that AIHA may precede the onset of NHL by months or years [32].The strength of the DAT does not always predict the biological activity of antibodies. However, substantial studies tend to support a direct correlation between the strength of the DAT result and hemolysis [33].
In order to define the spectrum of underlying malignancies and analyze the chronology of their occurrence with the occurrence of positive-DAT, we have conducted a retrospective study of all consecutive patients with positive DAT due to warm autoantibodies diagnosed and treated in our institution over an 11-year period.
Patients and Methods
Patient selection
All consecutive patients above 19 years of age with positive- DAT between 2009 and 2020 were selected for this study after an institutional review board approval. The DAT was performed upon physician request if hemolysis was suspected or in case of positive auto control in antibodies identification. The diagnosis of AIHA was made if the hemoglobin level was below 12 g/dl in men and 11 g/dl in women, the haptoglobin level was below 0.34 g/l and spherocytosis were present on blood smear.
Epidemiological, clinical and biological data for each patient were collected and recorded in a study specific case report form. This group of patients constituted a cohort and subjected to a long term follow up.
Underlying malignancies were especially looked for pathology report and bone marrow studies were reviewed. Patients who were receiving specific therapy were classified as having “active” disease. Collagen disorder and Systemic Lupus Erythematous (SLE) were diagnosed using specific serology tests. All positive warm DAT cases were included regardless of drug intake.
Two groups of positive-DAT patients were compared, the first group included those without AIHA and the second one included all cases with AIHA.
Positive-DAT may be due to the presence of alloantibodies in recently transfused patients. No patient had received blood product transfusion within the last 15 days before the IAT and DAT were carried out, therefore any allo-immune reaction could be excluded in our series.
Methods and statistical analysis
In routine pre-transfusion testing in our institution, ABO grouping, Rh typing, antibody screening and a cross match are performed. If the antibody screening is positive, then Antihuman Globulin (AHG) crossmatch is performed using selected donor units known to be negative for the corresponding antigens. In patients with pan-reactive panel and a positive auto-control, DAT is automatically performed.
The gel technique using BIO-RAD ID-Card “DC-Screening I” consisting of five different mono-specific AHG reagents; anti-IgG, anti-IgA, anti-IgM, anti-C3c (all rabbit) and anti-C3d (Monoclonal cell line C139-9) suspended in gel and the negative control was used for DAT. Antibody screening was done with commercial 3 cell panel (ID Dia cell-I-II-III Asia) gel card. The complete phenotype was determined either at diagnosis or subsequently within 3 weeks after corticosteroid treatment (D, C, E, c, e, Cw, K, k, Kpa, Kpb, Jsa, Jsb, Jka, Jkb, Fya, Fyb, Lea, Leb, P1, M, N, S, s, Lua, Lub and Xga); WBC-reduced donor RBCs matched with the patient's phenotype were provided for transfusion.
To determine whether positive-DAT occurred more frequently in patients with a specific disease or whether these were chance associations, contingency tables were drawn up for every underlying disease encountered in this series. The data were subjected to statistical analysis using chi squared (χ2) test with Yates correction at one degree of freedom; the significance level was set at p<0.05.
Results
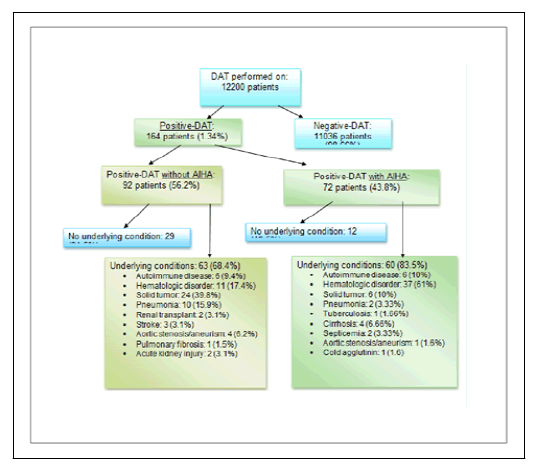
12,200 patients were subjected to testing, 164 patients (1.34%) were found to have a positive-DAT with warm autoantibodies. Seventy two patients (43.8%) had AIHA, 28 of them (40%) had hemoglobin level below 7.5 g/dl. The median age was 62 years (range: 19 to 86 years). There were 77 females and 86 males. The median follow-up time was 90 months (18 to 132 months).
The majority of patients with AIHA (83.5%) had underlying disease versus 68% of those without AIHA. Twenty nine patients (15.5% of positive-DAT patients) had no AIHA and no underlying disease whereas only 12 patients (7% of positive-DAT patients) had AIHA but no apparent underlying condition.
Hematologic disorders of both lymphoid and myeloid origin, autoimmune diseases and solid tumors were respectively found in 24.4%, 18.3% and 11% of positive-DAT patients (Figure 1).
Although, many patients had concomitantly two different conditions the most frequently observed hematological condition was MDS (17 cases), followed by MM (8 cases), NHL (6 cases), CLL (5 cases), MPN (5 cases) and AML (4 cases) as shown in Table 1 with the chronology of appearance.
| Chronology of DAT occurrence | |||||||
| Hematologic disorders | Diagnosis | AIHA | No AIHA | Total positive-DAT | Positive-DAT at presentation | Subsequent positive-DAT | |
| Myeloid disorders | MDS | 16 | 1 | 17 | 13 (76%) | 4 (23%) | |
| MDS | 10 | 1 | 11 | 8 (47%) | 3 (17.5%) | ||
| MDS+MPN | 2 | 0 | 2 | 1 (5.5%) | 1 (5.5%) | ||
| MDS+AML | 3 | 0 | 3 | 3 (18%) | 0 | ||
| MDS+CML | 1 | 0 | 1 | 1 (5.5%) | 0 | ||
| AML | 3 | 0 | 3 | 3 (100%) | 0 | ||
| MPN | 3 | 2 | 5 | 3 (60%) | 2 (40%) | ||
| Lymphoid disorders | MM | 4 | 4 | 8 | 1 (12.5) | 7 (87.5%) | |
| NHL | 3 | 3 | 6 | 5 (83%) | 1 (17%) | ||
| HD | 2 | 0 | 2 | 2 (100%) | 0 | ||
| ALL | 2 | 0 | 2 | 2 (100%) | 0 | ||
| CLL | 4 | 1 | 5 | 2 (40%) | 3 (60%) | ||
Table 1: Hematologic malignancy and the chronology of positive-DAT occurrence.
Table 2 shows the numbers of patients with the different underlying diseases compared to the total numbers of patients with the same condition treated in the institution during the same period.
| Disease category | Number (%) | Underlying disease | Positive DAT | AIHA | No AIHA | Number of inpatients (same period) | % |
|---|---|---|---|---|---|---|---|
| B-cell and Plasma cell malignancies | 23 (14%) | . | |||||
| Non-Hodgkin’s lymphoma | 6 | 3 | 3 | 195 | 3% | ||
| Hodgkin’s disease | 2 | 2 | 0 | 49 | 4% | ||
| Acute lymphoblastic leukemia | 2 | 2 | 0 | 12 | 15% | ||
| Chronic lymphocytic leukemia | 5 | 4 | 1 | 300+ | ND | ||
| Multiple myeloma | 8 | 4 | 4 | 79 | 10% | ||
| Myeloid disorders | 27 (10.3%) | ||||||
| Myelodysplastic syndrome | 17 | 16 | 1 | 207 | 8% | ||
| Acute-myelogenous leukemia | 4 | 4 | 0 | 70 | 5.60% | ||
| Chronic myelogenous leukemia | 1 | 1 | 13+ | ND | |||
| Myeloproliferative neoplasms | 5 | 3 | 2 | 57+ | ND | ||
| Malignant solid tumors | 30 (18.3%) | ||||||
| Breast cancer | 9 | 0 | 3 | 600+ | ND | ||
| Prostate cancer | 3 | 0 | 3 | 250+ | ND | ||
| Pancreatic cancer | 1 | 0 | 1 | 99+ | ND | ||
| Renal cell carcinoma | 2 | 45 | 4.50% | ||||
| Lung cancer | 1 | 0 | 1 | 290+ | ND | ||
| Gastric cancer | 1 | 0 | 1 | 60+ | ND | ||
| Bladder cancer | 1 | 0 | 1 | 100+ | ND | ||
| Colon cancer | 3 | 0 | 3 | 300+ | ND | ||
| Ovarian cancer | 1 | 0 | 1 | 60+ | ND | ||
| Liver cancer | 4 | 0 | 4 | 60+ | ND | ||
| Esophageal cancer | 3 | 1 | 2 | 30+ | ND | ||
| Soft tissue sarcoma | 1 | 0 | 1 | 30+ | ND | ||
| Autoimmune diseases | 18 (11%) | ||||||
| Systemic lupus erythematous | 6 | 29 | 20.50% | ||||
| Autoimmune hepatitis | 3 | 12 | 25% | ||||
| Small vessel vasculitis | 2 | ND | |||||
| Autoimmune myelofibrosis | 1 | ND | |||||
| Rheumatoid arthritis | 5 | 50 | 10% | ||||
| Autoimmune thyroiditis | 1 | 8 | ND | ||||
| Renal transplants | 2 (1.5%) | 2 | 0 | 2 | 25 | 8% |
Table 2: Underlying disease categories in positive-DAT patients and the significance of test with “Yates” correction for each category calculated according to the total.
Among the underlying solid tumors, only the renal cell carcinoma was significantly associated with the development of positive DAT.
The strength of DAT reaction was correlated with the presence of hemolysis in the entire cohort. A strong reaction (++ +/++++) was seen in 75% of MM, 50% of NHL and CLL and 33%of MDS patients. The DAT was also strong in patients with autoimmune disease particularly autoimmune hepatitis (2 patients), SLE (2/6 patients) and one patient with autoimmune myelofibrosis associated with diffuse large B-cell lymphoma.
Regarding the patterns of reactivity (Table 3). The most common pattern of reactivity was with anti IgG. The strength of reaction was correlated with the hemolysis. Most patients without evidence of hemolysis had a+ to ++strength of DAT reaction (Table 4). Speci ic treatment of the underlying disease with chemotherapy has signi icantly improved the severity of AIHA and lead to complete reversal of DAT in 70% of cases. Shows the allo-antibodies in alloimmunized, positive-IAT patients.
| DAT strength/Severity of hemolysis/Autoantibody | |||||||
| IgG | IgG+C3d | C3d | |||||
| Total Nb | Nb | Severe hemolysis | Nb | Severe hemolysis | Nb | Severe hemolysis | |
| + | 65 (40%) | 51 | 11 (21%) | 11 | 6 (55%) | 3 | 0 |
| ++ | 65 (40%) | 60 | 24 (40%) | 3 | 1 (33%) | 2 | 0 |
| +++ | 24 (14.5%) | 20 | 13 (65%) | 2 | 1 (50%) | 2 | 1 (50%) |
| ++++ | 9 (5.5%) | 4 | 4 (100%) | 4 | 4 (100%) | 1 | 1 (100%) |
Table 3: Strength of reactivity correlated with the erythrocyte auto-antibody type and the presence of severe hemolytic anemia (hemoglobin level ≤ 7.5 g/dl).
| Allo-antibody specificity in allo-immunized patient: | |||
| Anti-C | 3 | 9.70% | |
| Anti-D | 5 | 16% | |
| Anti-E | 5 | 16% | |
| Anti-Fya | 1 | 3% | |
| Anti-Jka | 1 | 3% | |
| Anti-Kell | 11 | 36% | |
| Anti-M | 5 | 16% | All biphasic and clinically significant |
Table 4: Alloantibodies identification in allo-immunized patients with positive-IAT and positive-DAT.
Clinical and biological characteristics of patients with positive DAT
Characteristics of patients are summarized in Table 5. Splenomegaly was reported in 26 patients (14.5%). Four patients (2.5%) had a splenectomy; one for congenital dyserythropoietic anemia, 2 for autoimmune cytopenia and one for traumatic splenic injury. Thrombocytopenia was present in 55 patients (34.5%) and was part of the autoimmune process (Evan’s syndrome) in 6 cases.
| Clinical and biological characteristics | |
| Mean age (ranges) | 62 years [19-86 years] |
| Sex; ratio | 77 females; 86 males |
| Underlying disease | 124 (76%) |
| No underlying disease | 39 (24%) |
| History of splenectomy | 4 (2.5%) |
| Reported splenomegaly | 26 (14.5%) |
| Associated thrombocytopenia | 55 (34.5%) |
| Associated neutropenia | 41 (25.5%) |
| IgG | 130 (80%) |
| C3d | 10 (6%) |
| C3d&IgG | 19 (11%) |
| IgA | 2 (1.5%) |
| Positive DAT&Positive IAT | 31 (19%) |
Table 5: Demographic, clinical and laboratory characteristics of DAT-positive patients. Positive DAT&
One hundred and twenty three cases (75%) were considered to be secondary to an underlying disease as shown in Table 1. Forty-one cases (25%) were considered as primary. Most of these patients had been admitted for surgical purpose. Among them thirteen patients (31%) were taking no medication and had no chronic disease (mean age 50 years, range: 19 to 72 years) and 28 patients (69%) had diabetes, coronary artery disease and renal failure (mean age 77 years, range: 50 to 81years). Forty percent of patients had no history of previous transfusion.
A hematologic non lymphoid disorder was found in 27 patients (10.3%) including myeloproliferative neoplasm, myelodysplastic syndrome and acute myeloblastic leukemia. Bcell and plasma cell malignancy were found in 23 patients (14%). Active solid tumor under specific treatment was found in 30 patients (18.3%).
Positive-DAT was present in 76% of MDS patients at presentation while 87% of MM patients developed positive-DAT subsequently during treatment and follow-up.
All patients with MPN were treated with hydroxyurea. However most of them were treated as outpatients and rarely tested for positive-DAT.
Our results showed a significant association between the occurrence of positive-DAT and MM, MDS and AID. However no significant association was found with solid malignant tumors apart from renal cell carcinoma.
The types of autoantibodies identified were as follows: IgG: 80%, C3d: 10%, both IgG and C3d: 19% and IgA (1.5%).
Patients with underlying lymphoma were more likely to be associated with erythrocyte coated-IgG and -C3d autoantibodies (605) when compared to multiple myeloma patients who were more likely to develop IgG only erythrocyte autoantibody (100%). C3d only coated RBCs was found in patients with SLE or without underlying disease.
Thirty-one patients (18.5) with positive DAT were IAT reactive. No patient had received blood product transfusion within the last 15 days before the IAT and DAT were carried out. The IAT were positive in 58% of patients without AIHA and 42% with hemolysis. A complete phenotype was determined in all 31 cases (Table 4 allo-antibodies) and these patients received prophylactic antigen matched-RBCs and white blood cellreduced transfusion (mean: 4 units/patients). Patients with AID and NHL were more likely to develop alloantibodies than patients with MM or MPN. Although, all the 4 MDS patients who developed positive-DAT subsequently had a history of multiple transfusions, only 2 of them (50%) had alloantibodies identified. Table 5 summarizes the alloantibodies detected at the first attempt or after 2 to 3 weeks of corticosteroid treatment by blood group.
All patients with AIHA received high dose corticosteroids (1.5 mg/kg/day) and 2 refractory cases with MDS and myelofibrosis received rituximab.
No patients developed malignancy subsequently to the diagnosis of positive-DAT however; the subsequent occurrence of a positive-DAT was an indicator of disease progression and refractoriness to treatment, especially in MDS patients.
Discussion
To better define the clinical spectrum of adult positive-DAT we have analyzed the data from 164 patients with a positive DAT out of 12,200 tests performed over an 11 year-period in our institution.
It is known that AIDs are associated with or preceding the development of lymphoproliferative diseases. The DAT might be positive at some time during the course of CLL in up to 35% of cases and could be an adverse prognostic factor in stage A [14,15].
The prevalence of CLL for the same period in our institution was difficult to evaluate because of the ambulatory basis of the follow-up, thus the positive-DAT proportion was difficult to estimate. However; 3% of NHL patients, 15% of ALL patients and 10% of MM patients had positive-DAT. Seven of the 8 MM patients had clinical and biological evidence of hemolysis regardless of the bone marrow infiltration by the plasma cells. AIHA was considered as a rare manifestation in patients with MM furthermore DAT may be falsely positive in IgG multiple myeloma due to the cross-reactivity with the monoclonal Ig or in patients treated with anti-CD38 [21].
Case series identified wide spectrum of autoimmune disorders, seemingly unrelated, in patients with MDS [22-30]. A positive-DAT was found in approximately 8% of MDS patients with IgG and or C3 on the RBCs which is in concordance with our results [28]. Patients with early MDS; RA and RARS show a higher incidence of anti-erythrocyte allo-and auto- antibodies and manifest overt non-organ specific autoimmune disorders. Furthermore, MDS share some of the features of aplastic anemia, a disease with an established autoimmune pathogenesis and 10% to 20% of patients with MDS present with Autoimmune Disease (AID), which can be challenging to recognize[29,30].
A positive-DAT was found in 19% of agnogenic myeloid metaplasia cases but also, less frequently in polycythemia vera. In AML the incidence of positive-DAT was found to be about 11% with only C3 on RBCs and Anti-I elute specificity [30,31]. Our series includes 4 AML cases with no auto- or alloantibody specificity.
Post-transplant immune-mediated hemolysis with positive- DAT is a known phenomenon that may be due to the immunosuppressive therapy that leads to an unbalanced B and T cell lymphopoiesis and the development of RBC autoantibodies.
Little has been reported about AIHA preceding the development of solid tumor [37-40]. Subsequent development of malignancy in patients with AIHA has rarely been addressed outside of scattered case studies [40]. Positive-DAT in blood donors indicates the presence of warm-reactive autoantibodies without evidence of hemolysis. An increased risk of malignancy has been reported among donors with positive-DAT [40]. Cases of paraneoplastic AIHA in solid tumor have been reported. Puthenparambil, et al. [38] reviewed 52 of them and found that AIHA may occur prior to, concurrent with and well after the treatment of cancer, 70% of the patients had warm autoantibodies and 30% cold antibodies, some patients had multi-lineage (Evans syndrome) or even multiorgan autoantibodies and it may occur in every type of cancer but more commonly in renal cell carcinoma which is in agreement with our results. Paraneoplastic manifestations may lead to an early detection of cancer and therefore be a favorable prognostic factor but also it may indicate a recurrence or an advanced stage.
Non-malignant conditions found to be significantly associated with positive-DAT: Pneumonia, aortic stenosis and aneurism, liver cirrhosis and heavy alcoholism, TB, pulmonary fibrosis and EBV infection in acute phase with high serum IgM.
Patients with a positive-DAT differ widely with respect to clinical manifestation; they may have no sign of hemolysis or may present severe hemolysis. A positive-DAT does not distinguish conclusively autoantibodies of clinical importance from those without [32-35].
IgA autoantibodies were detected in two of our patients (1.5%), IgG was detected in 130 patients (80%), IgG and C3d in 19 patients (11%) and C3d in 10 patients (6%) [25,35]. These results are in agreement with published data however IgA are reported to be present in 14% of warm-type AIHA, almost always associated with IgG and/or IgM antibodies [30].
In our study, the DAT reactivity was stronger in patients with AID than those with hematologic malignancy in whom the degree of anemia was also dependent on the bone marrow ability to produce RBCs. The strength of DAT reaction was also correlated with the severity of hemolysis. This correlation has been reported in some studies but was not found in others [33].
Thrombocytopenia was found in 34.5% of our cases, most of them were because of bone marrow failure (MDS, myelofibrosis) and 6 cases were considered as secondary Evans syndrome. The mechanism by which autoimmune thrombocytopenia develops may be similar to AIHA. There is no “gold standard” test that can reliably establish the diagnosis of ITP. Response to corticosteroid therapy or intravenous immunoglobulin is supportive of the diagnosis but a positive response does not exclude secondary ITP [34,35]. Evans syndrome may show or precede a variety of underlying diseases which may influence both the management and the outcome [36]. Positive-DAT has been found in 22% of ITP cases even without apparent anemia [39].
Studies on monospecific antiglobulin reactions have failed to attribute a particular underlying disease to a specific autoantibody [32-35]. The presence of IgG only is more commonly seen in drug-induced autoimmune hemolysis, complement only may reflect a more severe condition and both may be associated with the occult form or the apparent severe hemolysis. No disease specificity for auto- or alloantibody was found in our study.
In our study, alloantibodies were identified in 31 cases (19%). Alloantibodies against RBCs have been shown to be present in a large proportion (25%-47%) of sera from patients with AIHA [33,41]. A complete phenotype for patients with warm autoantibodies is very helpful for the blood bank to provide prophylactic antigen-matched donor RBCs for these patients who are at high risk for alloimmunization and delayed hemolytic transfusion reactions. All 5 cases of Anti-M antibody identified were biphasic and considered as clinically significant. All these patients received “M”-antigen negative blood transfusion and no delayed hemolytic reaction was observed.
Our study sheds some light on the significance of AIHA and its relationships with underlying inflammatory or neoplastic conditions. A more precise definition of the spectrum of underlying diseases may lead to a better understanding of DAT positivity and to elaborate a surveillance plan or a screening program for malignancy in these patients.
Despite the retrospective design of the study and the small size of this cohort that allow few statistically significant conclusions, the analysis of the data may point out a new insight into the spectrum of associated malignant conditions.
Conclusion
A positive-DAT with or without AIHA suggests the presence of underlying malignancy, mainly hematological and even in early stages. All patients with MDS should be tested for DAT in diagnostic and therapeutic intent.
References
- Bohnen RF, Ultmann JE, Gorman JG (1968) The direct coombs test: Its clinical significance. Ann Intern Med 68: 19-32.
[Crossref], [Google Scholar], [Indexed]
- Huh YO, Lichtiger B (1985) Evaluation of a positive autologous control in pretransfusion testing. Am J Clin Pathol 84: 632-636.
[Crossref], [Google Scholar], [Indexed]
- Toy PT, Chin CA, Reid ME (1985) Factors associated with positive direct antiglobulin tests in pretransfusion patients: A case-control study. Vox Sang 49: 215-220.
[Crossref], [Google Scholar], [Indexed]
- Rochant H (1980) Hemolytic anemias with a negative Coombs' test and a positive Coombs' test without hemolytic anemia. Ann Med Interne 131: 452-466.
[Google Scholar], [Indexed]
- Habibi B, Muller A, Lelong F (1980) Red cell autoimmunization in a "normal" population. 63 cases author's transl. Nouv Presse Med 9: 3253-7.
[Google Scholar], [Indexed]
- Dausset J, Colombani J (1959) The serology and the prognosis of 128 cases of autoimmune hemolytic anemia. Blood 14: 1280-301.
[Crossref], [Google Scholar], [Indexed]
- Sokol RJ, Hewitt S, Stamps BK (1981) Autoimmune Haemolysis: An 18-year study of 865 cases referred to a regional transfusion centre. Br Med J 282: 2023-7.
[Crossref], [Google Scholar], [Indexed]
- Worlledge SM, Blajchman MA (1972) The autoimmune haemolytic anaemias. Br J Haematol 23: 61-9.
- Eyster ME, Jenkins DE (1969) Erythrocyte coating substances in patients with positive direct antiglobulin reactions. Correlation of gamma-G globulin and complement coating with underlying diseases, overt hemolysis and response to therapy. Am J Med 46: 360-71.
[Crossref], [Google Scholar], [Indexed]
- Dacie JV, Worlledge SM (1969) Auto-immune hemolytic anemias. Prog Hematol 6: 82-120.
- Leddy JP, Bakemeier RF (1967) A relationship of direct Coombs test pattern to autoantibody specificity in acquired hemolytic anemia. Proc Soc Exp Biol Med 125: 808-11.
[Crossref], [Google Scholar], [Indexed]
- Petz LD (2004) Review: Evaluation of patients with immune hemolysis. Immunohematology 20: 167-76.
[Google Scholar], [Indexed]
- Rosenthal MC, Pisciota AV, Komninos ZD, Goldenberg H, Dameshek W (1955) The auto-immune hemolytic anemia of malignant lymphocytic disease. Blood 10: 197-227.
[Google Scholar], [Indexed]
- Ricci F, Tedeschi A, Vismara E, Colombo C, Veronese S, et al. (2013) Should a positive direct antiglobulin test be considered a prognostic predictor in chronic lymphocytic leukemia? Clin Lymphoma Myeloma Leuk 13: 441-6.
[Crossref], [Google Scholar], [Indexed]
- Quinquenel A, Nawakil CA, Baran-Marszak F, Eclache V, Letestu R (2015) Old DAT and new data: Positive direct antiglobulin test identifies a subgroup with poor outcome among chronic lymphocytic leukemia stage A patients. Am J Hematol 90: 5-8.
[Crossref], [Google Scholar], [Indexed]
- Polliack A, Lugassy G (1992) Autoimmunity and auto-immune syndromes associated with and preceding the development of lymphoproliferative disorders. Leukemia 4: 152-154.
[Google Scholar], [Indexed]
- Jachiet V, Mekinian A, Carrat F, Grignano E, Retbi A, et al. (2018) Autoimmune manifestations associated with lymphoma: Characteristics and outcome in a multicenter retrospective cohort study. Leuk Lymphoma 59: 1399-1405.
[Crossref], [Google Scholar], [Indexed]
- Jonsson U, Hansen-Pruss OC, Rundles RW (1950) Hemolytic anemia in myelogenous leukemia with splenectomy. Blood 5: 920-924.
[Crossref], [Google Scholar], [Indexed]
- Gordon BR, Coleman M, Kohen P, Day NK (1981) Immunologic abnormalities in myelofibrosis with activation of the complement system. Blood 58: 904-910.
[Crossref], [Google Scholar], [Indexed]
- Rondeau E, Solal-Celigny P, Dhermy D (1983) Immune disorders in agnogenic myeloid metaplasia: Relations to myelofibrosis. Br J Haematol 53: 467-475.
[Crossref], [Google Scholar], [Indexed]
- Dalal BI, Collins SY, Burnie K, Barr RM (1991) Positive direct antiglobulin tests in myeloma patients. Occurrence, characterization, and significance. Am J Clin Pathol 96: 496-499.
[Crossref], [Google Scholar], [Indexed]
- Sokol RJ, Hewitt S, Booker DJ (1989) Erythrocyte autoantibodies, autoimmune haemolysis and myelodysplastic syndromes. J Clin Pathol 42: 1088-91.
[Crossref], [Google Scholar], [Indexed]
- Barcellini W, Zaninoni A, Imperiali FG, Boschetti C, Colombi M, et al. (2007) Anti-erythroblast autoimmunity in early myelodysplastic syndromes. Haematologica 92: 19-26.
[Crossref], [Google Scholar], [Indexed]
- Grignano E, Jachiet V, Fenaux P, Ades L, Fain O, et al. (2018) Autoimmune manifestations associated with myelodysplastic syndromes. Ann Hematol 97: 2015-2023.
[Crossref], [Google Scholar], [Indexed]
- Barcellini W, Zaninoni A, Imperiali FG, Boschetti C, Colombi M, et al. (2007) Anti-erythroblast autoimmunity in early myelodysplastic syndromes. Haematologica 92: 19-26
[Crossref], [Google Scholar], [Indexed]
- Barcellini W, Giannotta JA, Fattizzo B (2021) Autoimmune complications in hematologic neoplasms. Cancers 13: 1532.
[Crossref], [Google Scholar], [Indexed]
- Sigler E, Levene NA, Berrebi A (1990) Positive direct antiglobulin test in myelodysplastic syndrome. Am J Hematol 33: 80.
[Crossref], [Google Scholar], [Indexed]
- Mufti GJ, Figes A, Hamblin TJ (1986) Immunological abnormalities in myelodysplastic syndromes I. Serum immunoglobulins and autoantibodies. Br J Haematol 63: 143-147.
[Crossref], [Google Scholar], [Indexed]
- Enright H, Miller W (1997) Autoimmune phenomena in patients with myelodysplastic syndromes. Leuk Lymphoma 24: 483-489.
[Crossref], [Google Scholar], [Indexed]
- Barrett AJ, Saunthararajah Y, Molldrem J (2000) Myelodysplastic syndrome and aplastic anemia: Distinct entities or diseases linked by a common pathophysiology? Semin Hematol 37: 15-29.
[Crossref], [Google Scholar], [Indexed]
- Solal-Celigny P, Vazeux R, Vroclans M, Amar M, Herrera A, et al. (1984) Positive Coombs test in acute leukaemia. Br J Haematol 57: 563-569.
[Crossref], [Google Scholar], [Indexed]
- Genty I, Michel M, Hermine O, Schaeffer A, Godeau B, et al. (2002) Characteristics of autoimmune hemolytic anemia in adults: Retrospective analysis of 83 cases. Rev Med Interne 23: 901-909.
[Crossref], [Google Scholar], [Indexed]
- Wheeler CA, Calhoun L, Blackall DP (2004) Warm reactive autoantibodies: Clinical and serologic correlations. Am J Clin Pathol 122: 680-685.
[Crossref], [Google Scholar], [Indexed]
- Alwar V, Shanthala DA, Sitalakshmi S, Karuna RK (2010) Clinical patterns and hematological spectrum in autoimmune hemolytic anemia. J Lab Physicians 2: 17-20.
[Crossref], [Google Scholar], [Indexed]
- Wikman A, Axdorph U, Gryfelt G, Gustafsson L, Björkholm M, et al. (2005) Characterization of red cell autoantibodies in consecutive DAT-positive patients with relation to in vivo haemolysis. Ann Hematol 84: 150-158.
[Crossref], [Google Scholar], [Indexed]
- Michel M, Chanet V, Dechartres A, Morin AS, Piette JC, et al. (2009) The spectrum of Evans syndrome in adults: New insight into the disease based on the analysis of 68 cases. Blood 114: 3167-3172.
[Crossref], [Google Scholar], [Indexed]
- Sokol RJ, Stamps R, Booker DJ (2002) Post transplant immune-mediated hemolysis. Transfusion 42: 198-204.
[Crossref], [Google Scholar], [Indexed]
- Puthenparambil J, Lechner K, Kornek G (2010) Autoimmune hemolytic anemia as a paraneoplastic phenomenon in solid tumors: A critical analysis of 52 cases reported in the literature. Wien Klin Wochenschr 122: 229-236.
[Crossref], [Google Scholar], [Indexed]
- Ben-Izhak C, Shechter Y, Tatarsky I (1985) Significance of multiple types of antibodies on red blood cells of patients with positive direct antiglobulin test: A study of monospecific antiglobulin reactions in 85 patients. Scand J Haematol 35: 102-108.
[Crossref], [Google Scholar], [Indexed]
- Rottenberg Y, Yahalom V, Shinar E (2009) Blood donors with positive direct antiglobulin tests are at increased risk for cancer. Transfusion 49: 838-842.
[Crossref], [Google Scholar], [Indexed]
- Shirey RS, Boyd JS, Parwani AV (2002) Prophylactic antigen-matched donor blood for patients with warm autoantibodies: An algorithm for transfusion management. Transfusion 42: 1435-1441.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences